But it's GMP!
There are two types of ‘scientists’.
1)Those that worship hierarchy as a church of science
2)And those who understand hierarchy is a cancer to the scientific method.
Those who worship at the ‘appeal to authority’ altar, unquestionably defend what Pfizer does as truth. They would never lie and their data must be assumed to be truth as it was reviewed by the FDA/EMA. All of the relevant 3-letter cathedrals that feed on Pharma tithe via the PDUFA act of 1992 are to be 100% trusted as peer reviewed fact. Let’s have a look at what some of these churches had to say about Pfizer’s data. It is not all self congratulatory back patting.
I was recently forwarded an even larger EMA dump by Josh Guetzkow (@jackanapes.substack.com)
This one is 397 pages and I have toured through the parts related to the genomics to provide you some background on the objections the EMA listed. The reason for doing this is that the Pro-Vax crowd has been critiquing our work as it is not GMP and they claim the methods used by Pfizer “were GMP so GMP trumps non-GMP… you are wrong”.
Let’s put aside the fact that the EMA doesn’t run any experiments and has to take Pfizers data and assume it’s all honest. Even with that assumed honesty from a conflicted party that has been fined for lack of honesty in the past…. The EMA still finds many objections to the work. I have to commend the EMA for the thorough review. This is a lot of work.
“Not yet considered adequate to allow for a proper assessment”.
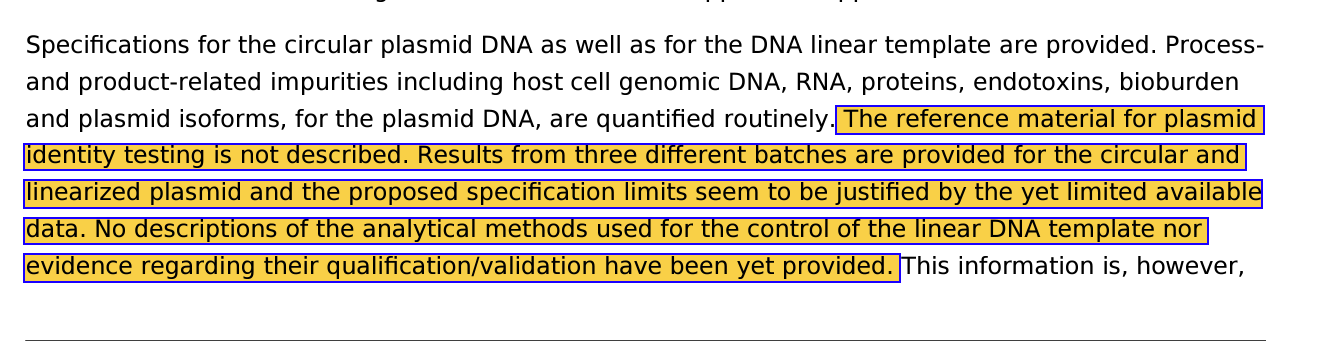
“No descriptions of the analytical methods used for the control of the linear DNA template”
This is where Blotgate is vindicated and much of the concerns voiced in our preprint come to light. The EMA has picked up on the fact that there is “no biological characterization is presented. This is not found acceptable”. Noted as a “Severe deficiency”.
“Characterization of BNT162b2 DS is currently not found acceptable” In Bold- “This is considered a major objection”.
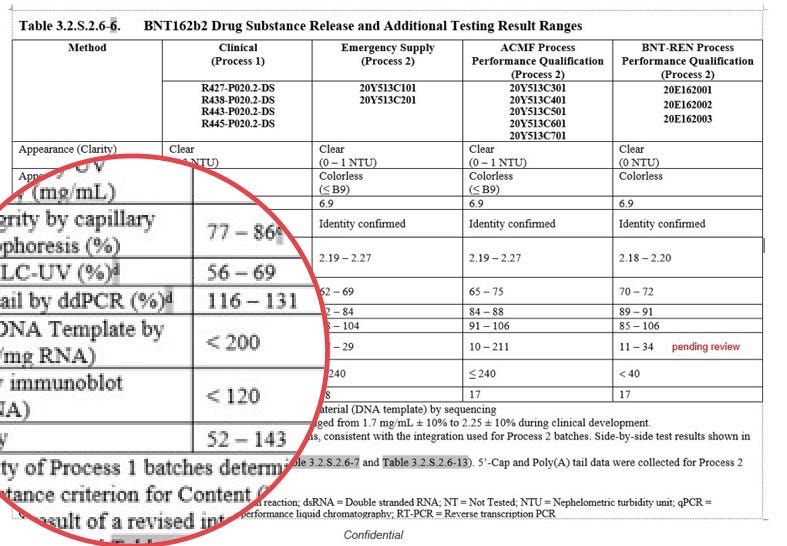
Residual DNA template contamination is noted by the EMA. They take note of this number varying by an order of magnitude in just a few lots. Odds are it varies even more if more lots are screened. This is the data Pfizer was able to cherry pick and hand to the EMA.
“Additional batch data are needed to conclude on the consistent removal of this impurity.”
We have been chastised for providing such data for free.
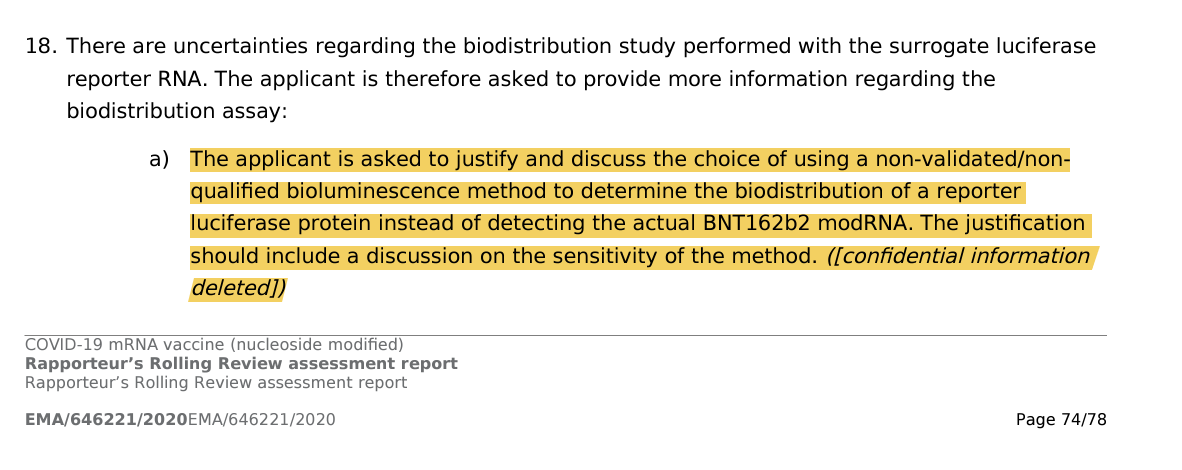
The bio distribution data is called out for using a bullshit luciferase model. Why didn’t they use the BNT162b2 mRNA as the model?
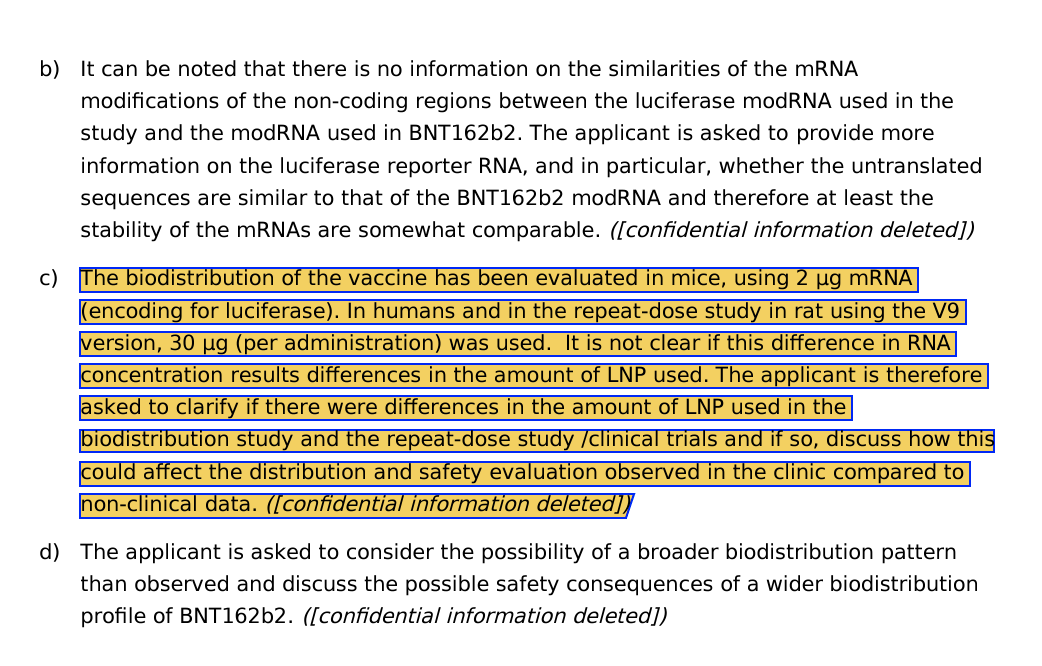
And no one likes the fact that they never used vehicle controls. They should inject mice with just LNPs and no mRNA to understand the toxicology of the independent parts.
Here is the zinger….
NOPE.
NOT GMP.
More concerns over lot to lot variation in the RNA integrity scores. Note the clinical trials already dosed people with these poor RNA integrity lots but found a way to yank those patients from the Interim Analysis.
“The company does not expect to have Process 2 included in the Final Analysis dataset.”
Whoops- give some poor lots out in your clinical trial and you can just delete them and their data like it never happened.
The EMA picks up on the fact that they provided sequence coverage for the transcript but not the plasmid. Recall, they generated this data with RNA-seq and found the plasmid.
WAIT? You mean the DNaseI step is variable? What could that lead to? Excess plasmid DNA in the vials? 211ng/mg is actually above one of the limits. I have been using the more forgiving limit of 330ng/mg but this document also has 200ng/mg listed in other parts of it.
They performed Next Gen sequencing but the methods are not well described.
CT values for the qPCR were hidden. Seems like a theme in C19.
The method used to linearize the plasmid details are missing.
BlotGate vindicated again by the EMA.
We wrote about Autoimmune concerns. That paper received 2 favorable reviews but was censored by the Journal editors.
This says everything. Their challenge studies are not convincing. This foreshadows the failure seen in the marketplace.
I wonder what confidential information is deleted?
They claim to have a qPCR method to monitor residual DNA. I have already critiqued this method for not listing the polymerase used or the CT value called. They also placed their qPCR assay in the T7→Kozak region which is the highest transcribed region of the plasmid. This is likely intentional as it will under-estimate the dsDNA present. They should have picked a non-transcribed region of the vector backbone as we did.
My major concerns with the document is that Pfizer used a very unconventional method to sequence the vaccines. They used an Oligo Mapping LC/MS method that is impossible to disentangle and floods the reviewer with pages of Mass Spec data and tables. No one does this today for DNA sequencing. The Next Gen Sequencing data exist but are largely hidden and only summarized. This is intentional as the next gen data will reveal the DNA contamination and the LC/MS method will not.
So when folks online assure you this is GMP grade, forward them this thread and ask them if they can address the major objections which caused the EMA to fail their GMP status.
Remind them of the recalls for Moderna in Japan. Visible metals in the vials is GMP?
Does this batch variation look like GMP to you?



























I blocked a commenter on their thread as they have been abusive and redundant.
They voiced 2 complaints.
Unless the vials are straight from Pfizer the whole study has custodial bias.
True. But straight from Pfizer has its own bias in that can cherry pick what they share.
We need to know what is in the field and Pfizer hasn’t been very religious on storage conditions for these new products. They changed the cold chain. The vials were sealed and showed no evidence of being tampered with.
The only question is age and RNA degradation.
The evidence against degradation is that the RNA integrity isn’t much worse than what Pfizer describes in public documents.
If the vials we degraded, we see worse RNA integrity than the TGA documents and we don’t see that.
2)second comment was attempting to be a spicey gotcha. The EMA doc above has a date of Nov. 2020. They claim this means Pfizer fixed everything after this.
What they fail to appreciate is the section that demonstrates Pfizer already had low RNA integrity lots in the trial and they make note of having to delete that data from the trial.
We also know the manufacturing for the trial was in flux between synthesized DNA and the migration to plasmids in ecoli.
So low quality shots made it into people and those results were omitted from the trial and the detractors of this work don’t care.
Well, that's embarrassing but not surprising. There was no way they could get these 'vaccines' out in a short time frame using proper manufacturing and distribution practices. I'm no biochemist, but I know enough about various disciplines of engineering to scoff at the claims of high quality manufacturing.
"The Next Gen Sequencing data exist but is largely hidden and only summarized."
Now that is bad news. Why didn't regulators ask for the results of modern sequencing? I've actually worked a little bit with reads from various machines, but as a software engineer building cloud computing infrastructure. Those files are large but I assume that there are a bunch of common tools that can be used to analyze them efficiently. You have to wonder about the regulators as well, since they allowed this.