Massachusetts has an OVERT lab shopping issue and it’s very evident in the now public data from MCR Labs.
To complement and confirm the MCR data, we received a new Public Records Request from the Massachusetts CCC confirming this trend.
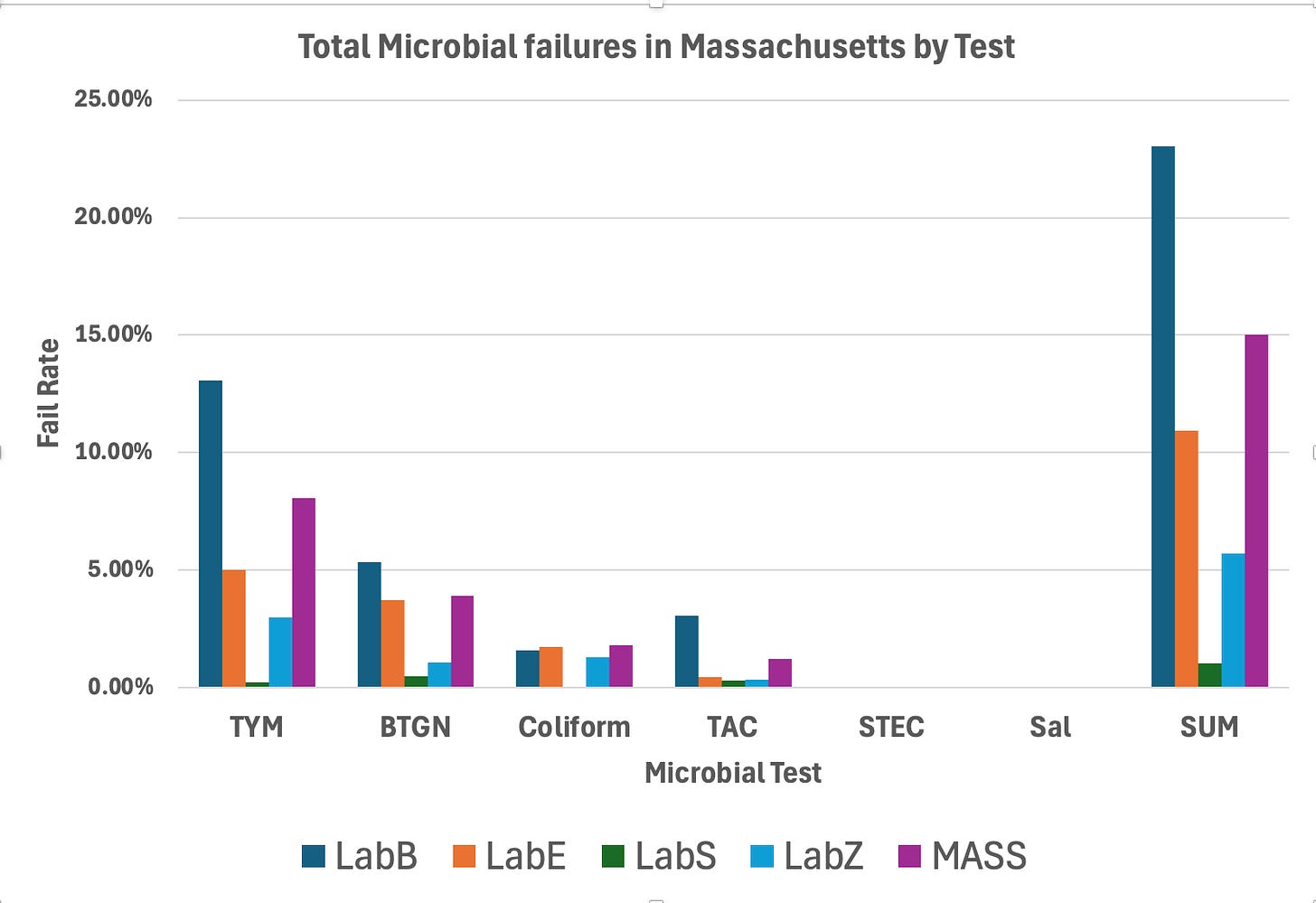
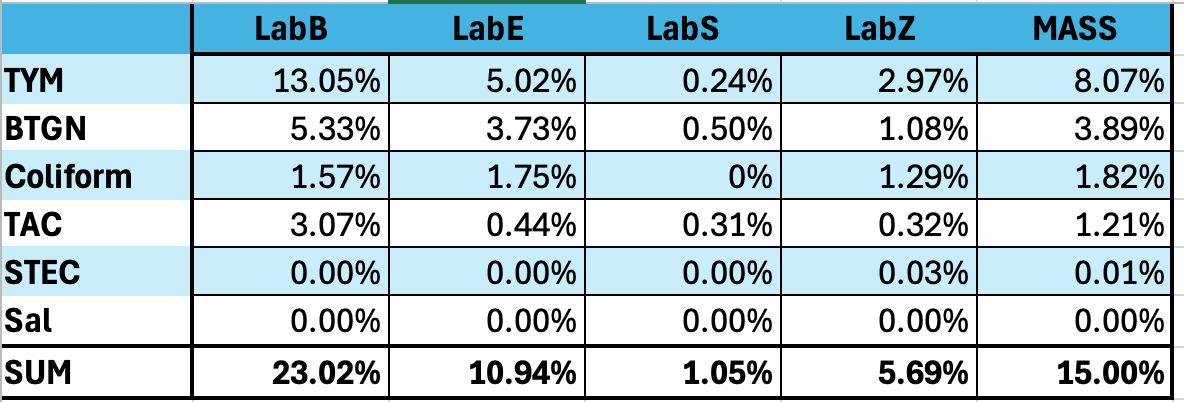
This dataset includes all six of the required microbial tests from the state of Massachusetts. In order of their respective fail rates they are…
TYM > BTGN > Coliform > TAC > STEC > & Salmonella.
Lets look at the fail rates between the labs.
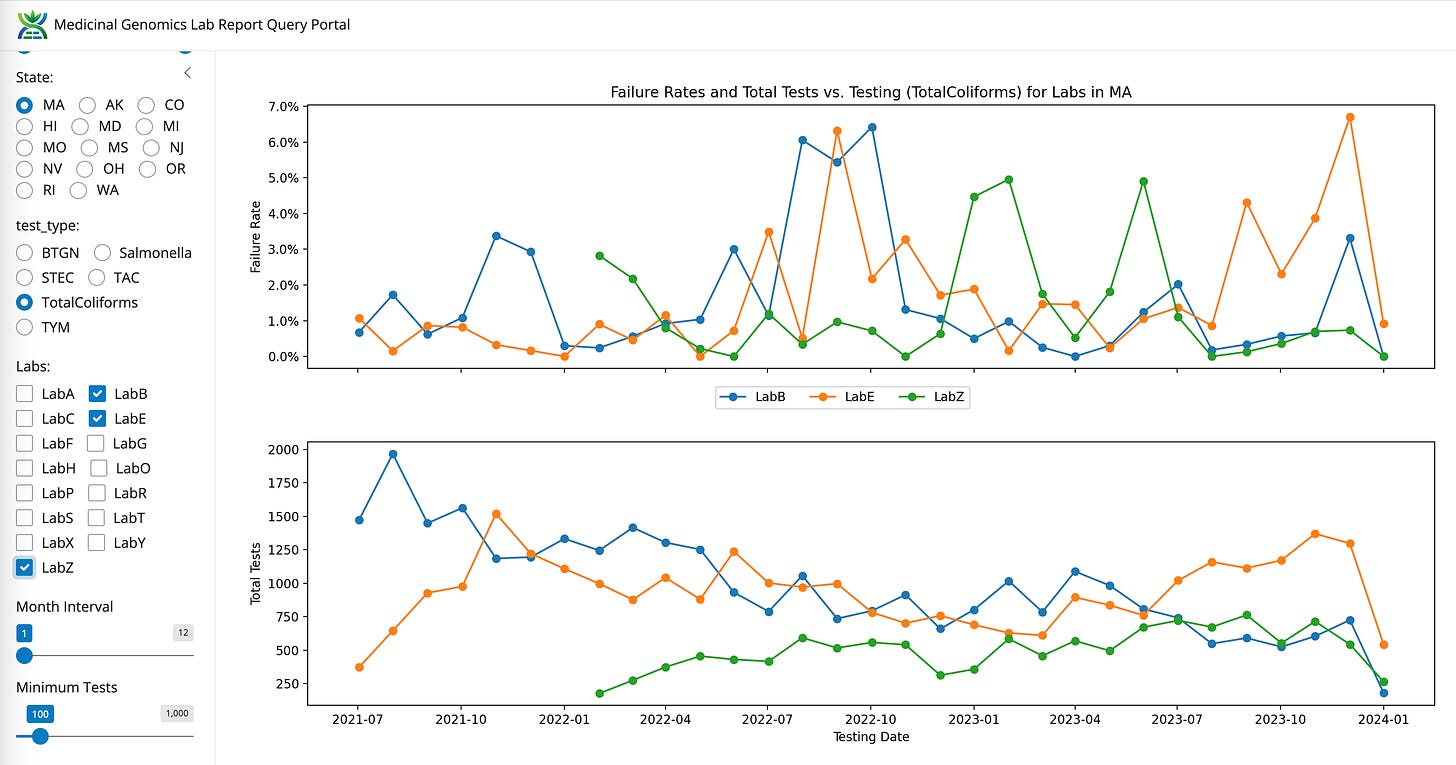
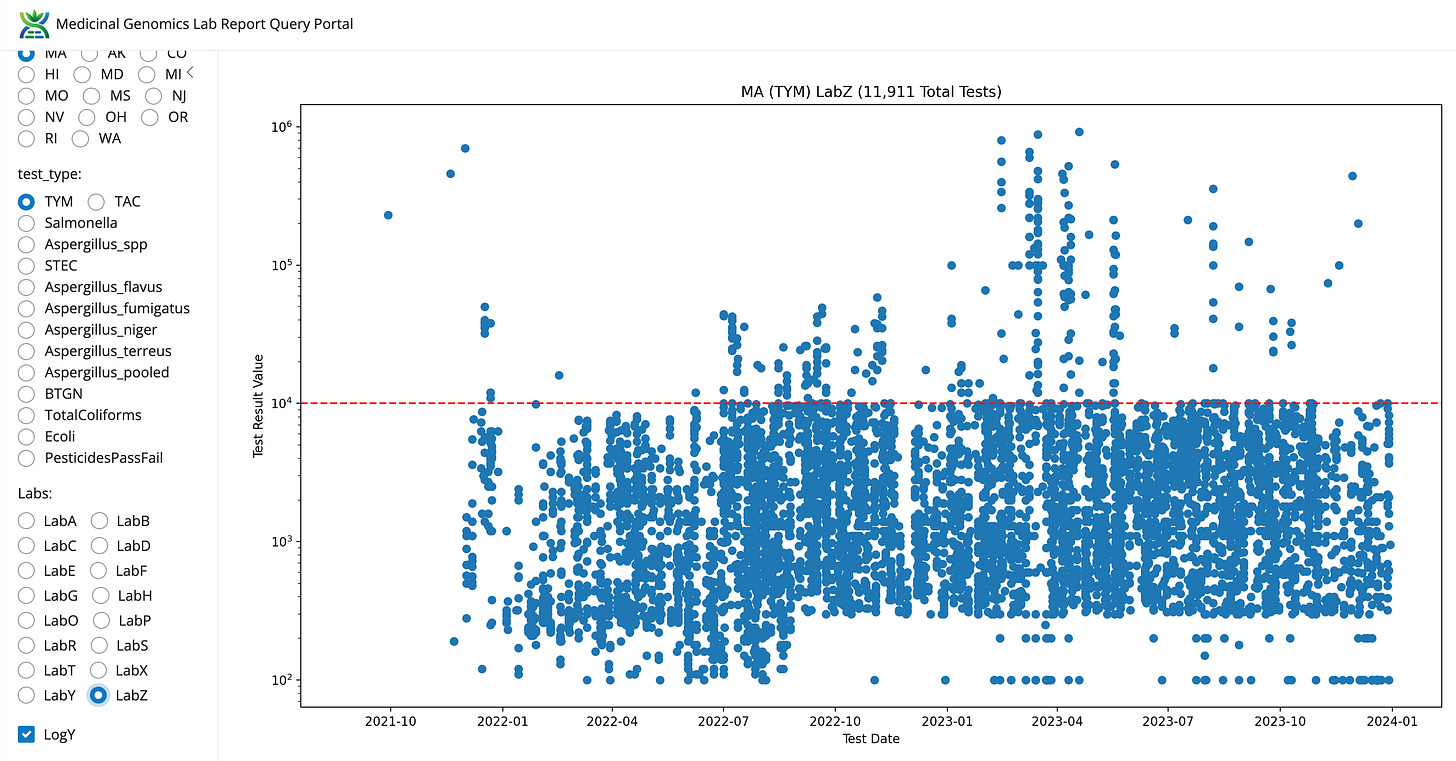
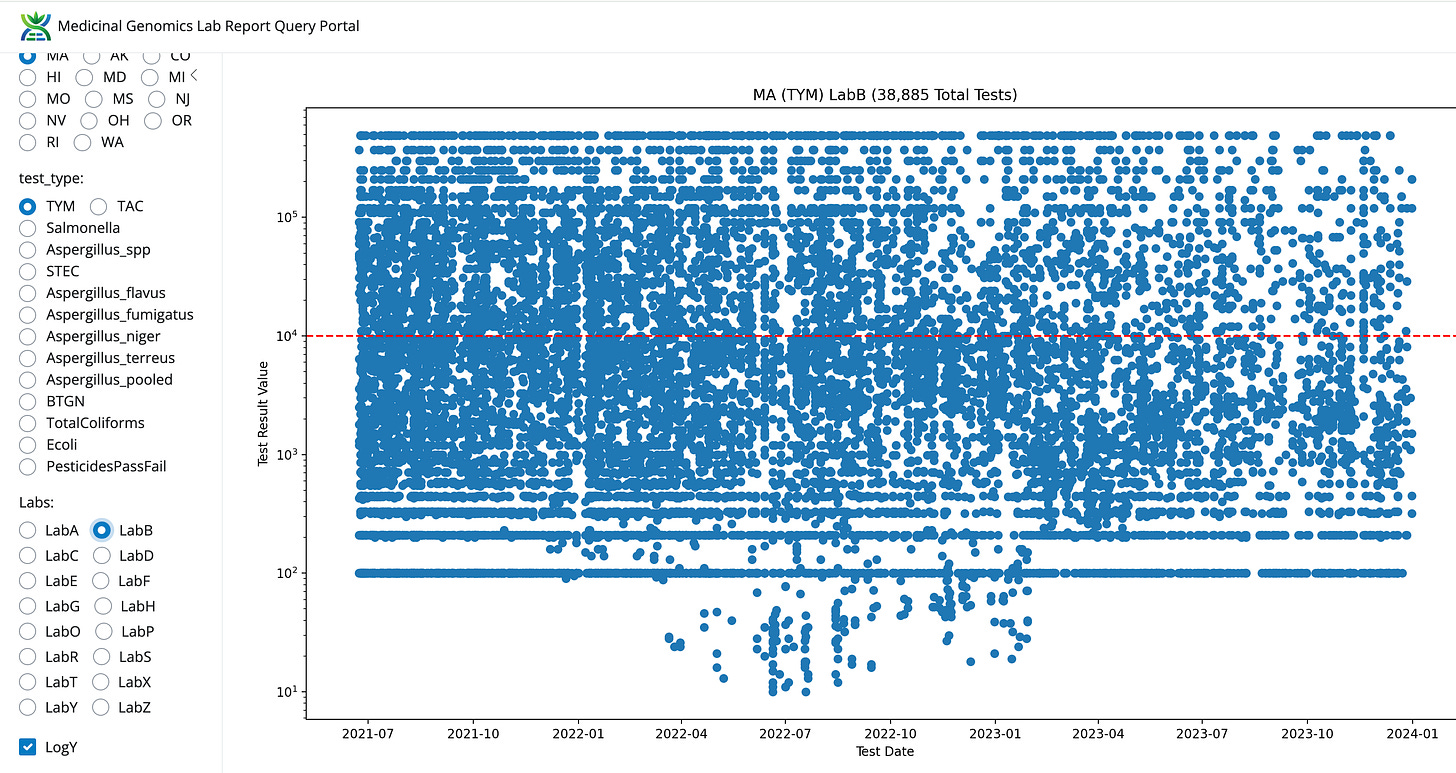
The most obvious lab shopping is occurring from the highest failure rate tests like TYM. LabZ is picking up lots of volume and LabB is losing test volume. This will be discussed more below. The next question this data can address is to what degree are the other 5 tests also contributing to lab shopping?
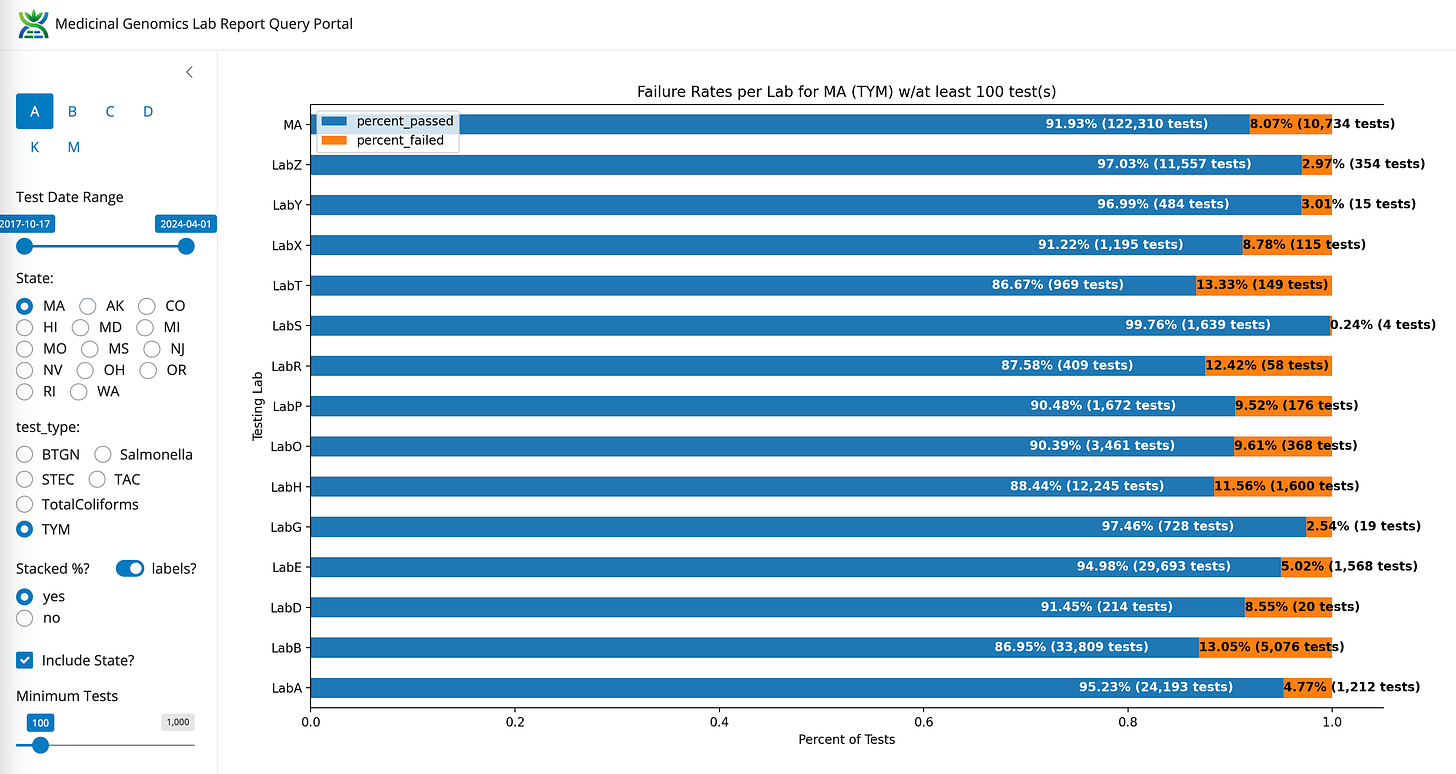
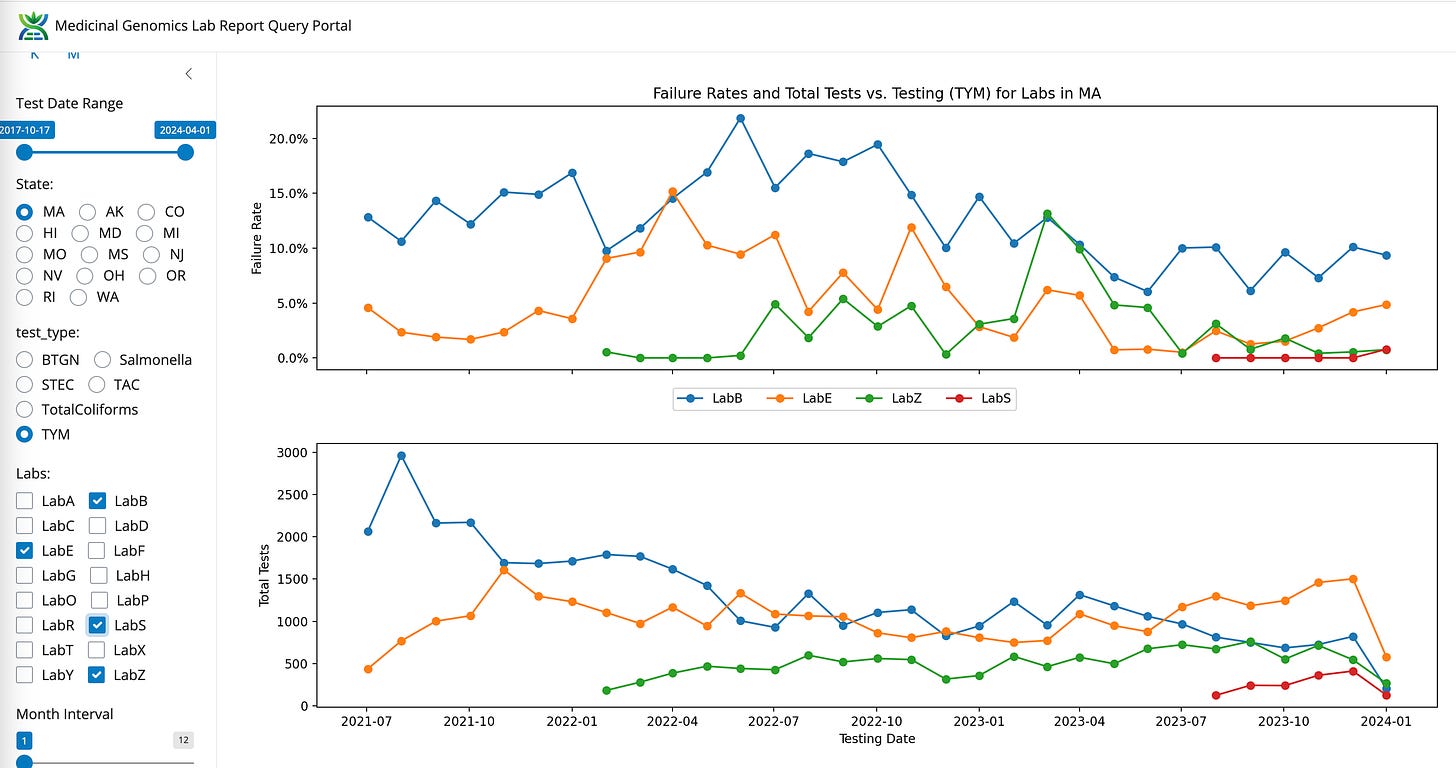
TYM- State Average Fail Rate (8.07%). Range 55 fold (0.24%-13.3%)
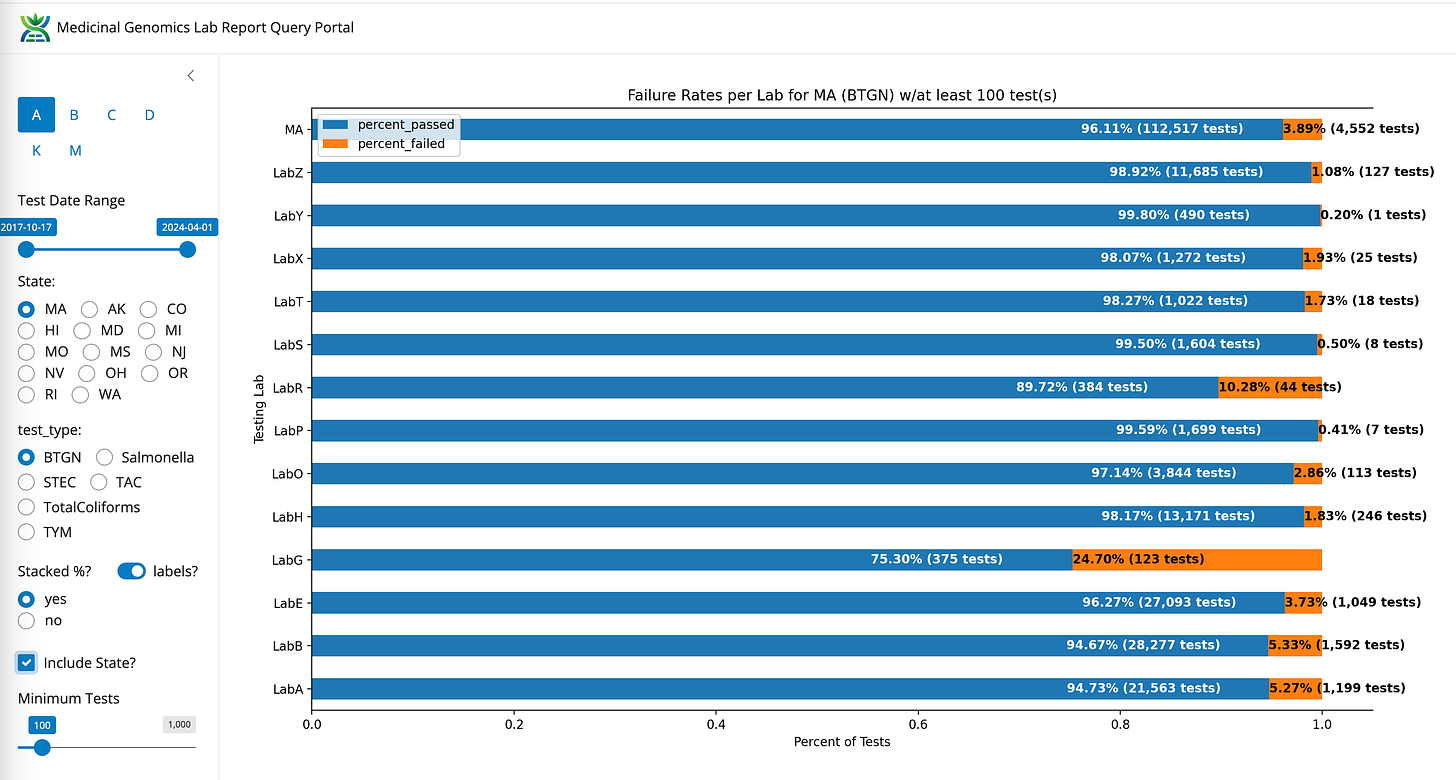
BTGN- State Average Fail Rate (3.89%). Range 123 fold (0.2%-24.7%)
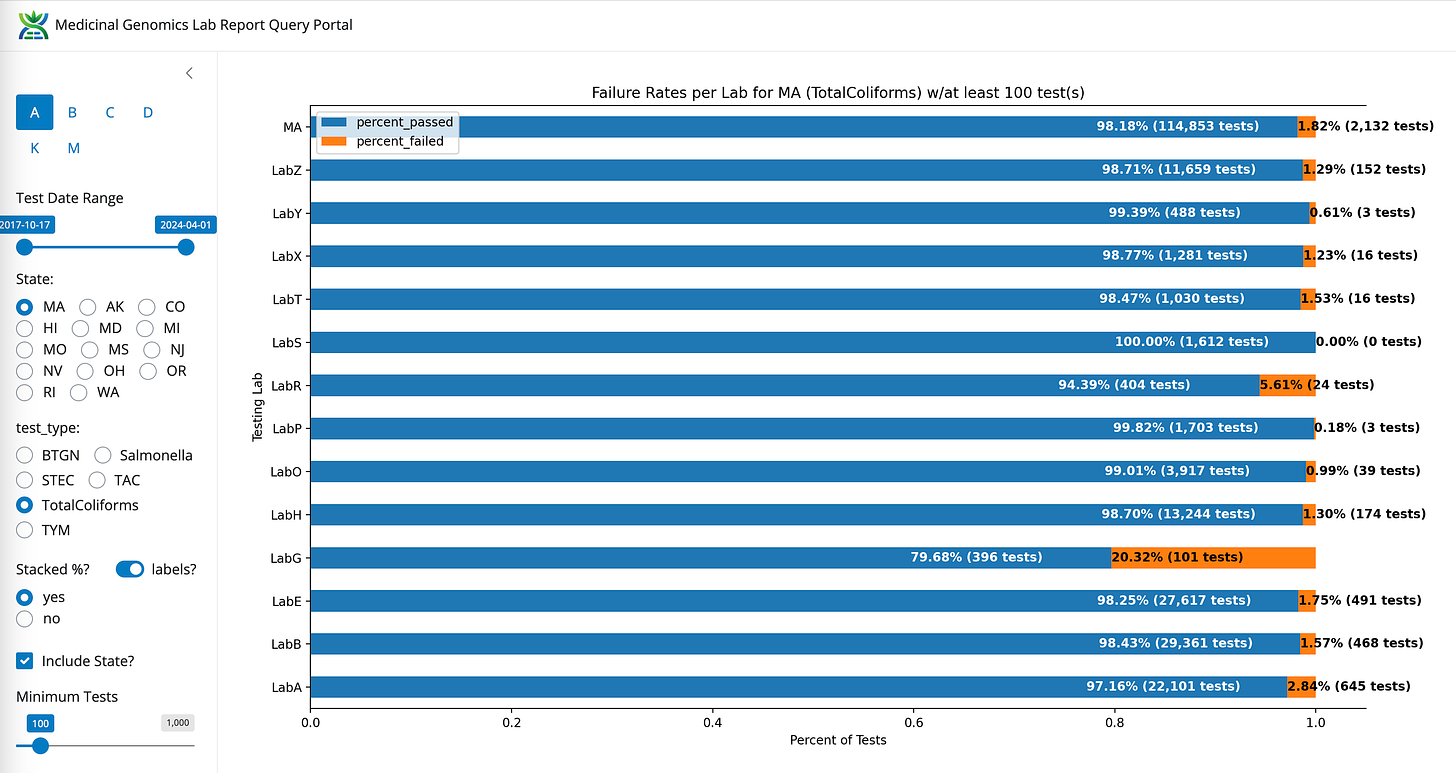
Coliform- State Average Fail Rate (1.82%). Range undefined (0%-20.32%)
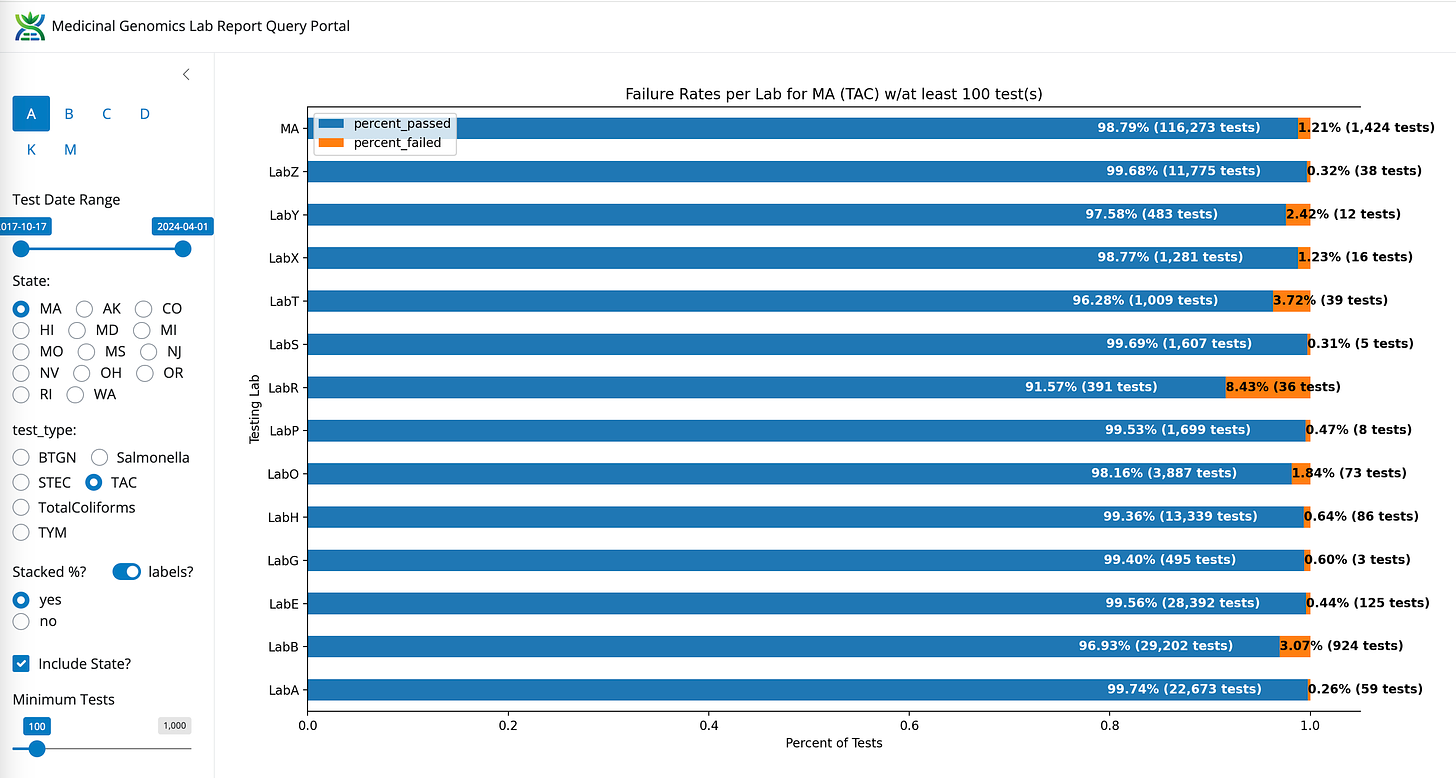
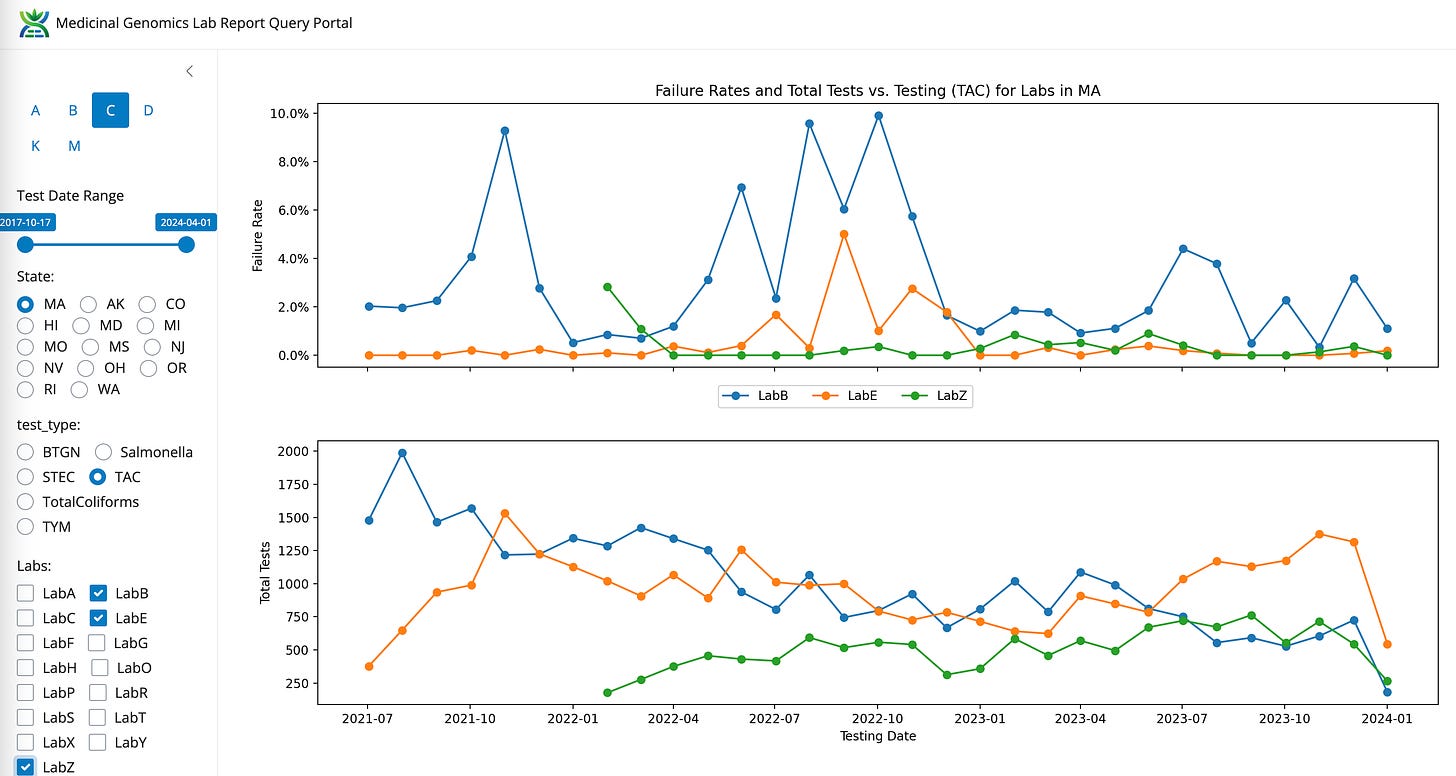
TAC- State Average Fail Rate (1.21%). Range 32 fold (0.26%-8.43%)
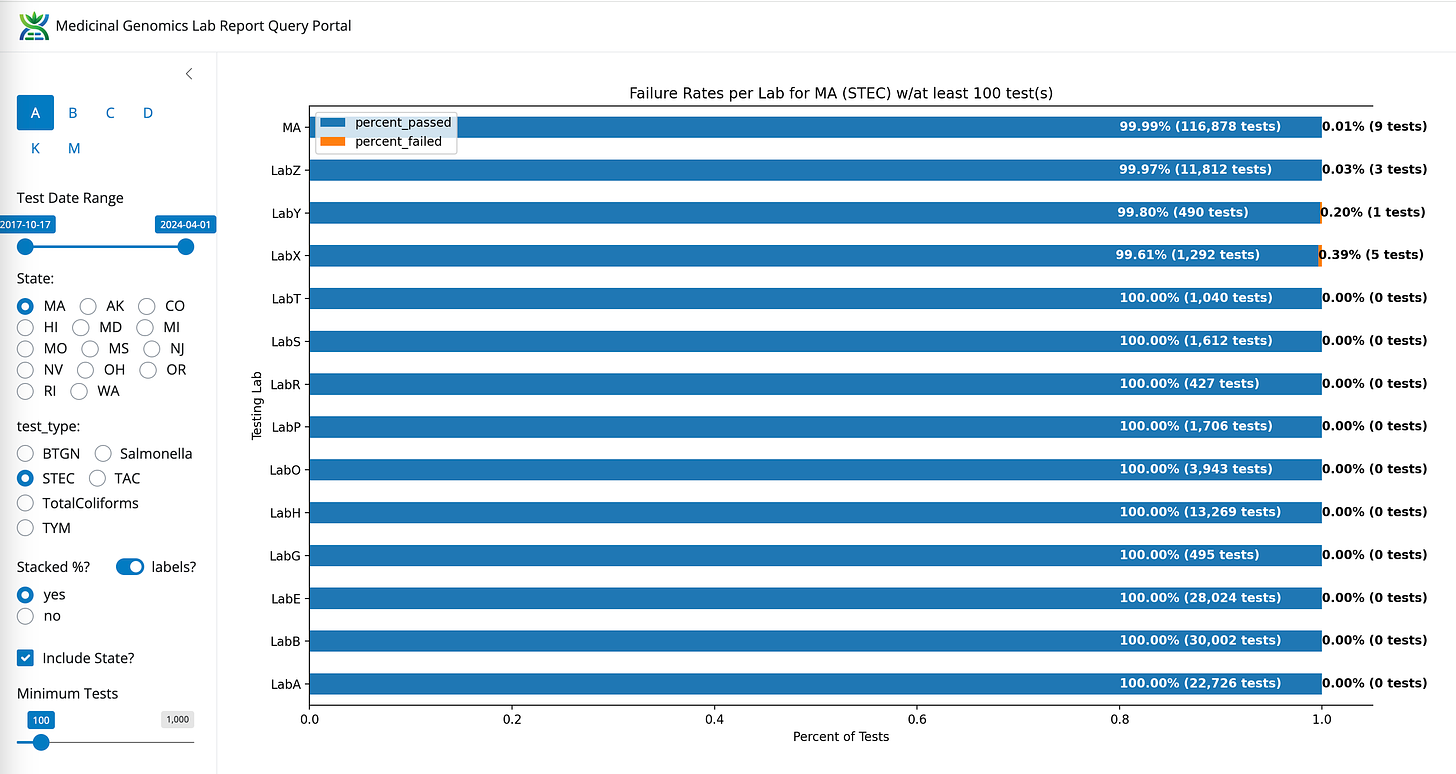
STEC- State Average Fail Rate (0.01%). Range undefined (0%-0.39%)
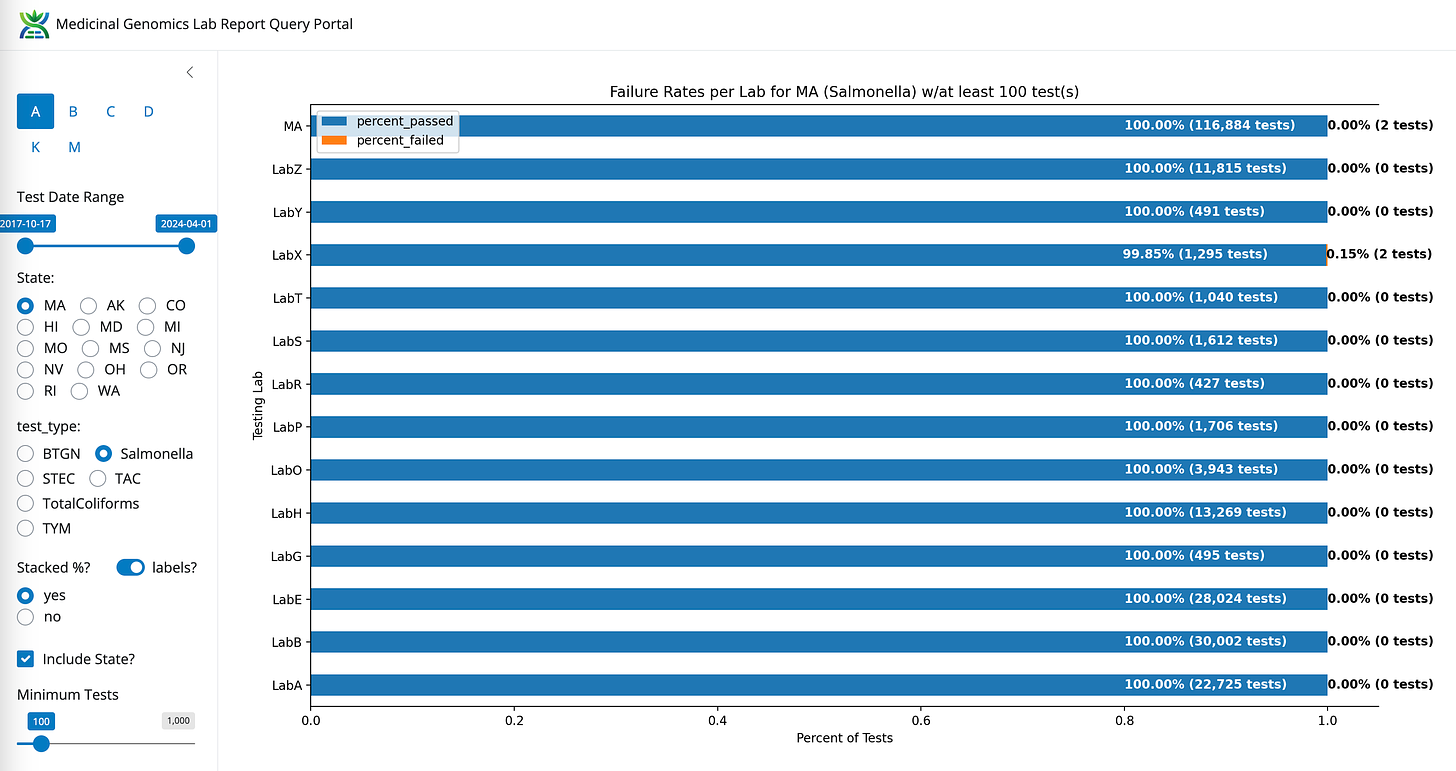
Salmonella- State Average Fail Rate (0.00% or 2/116,884). Range undefined (0%-0.15%)
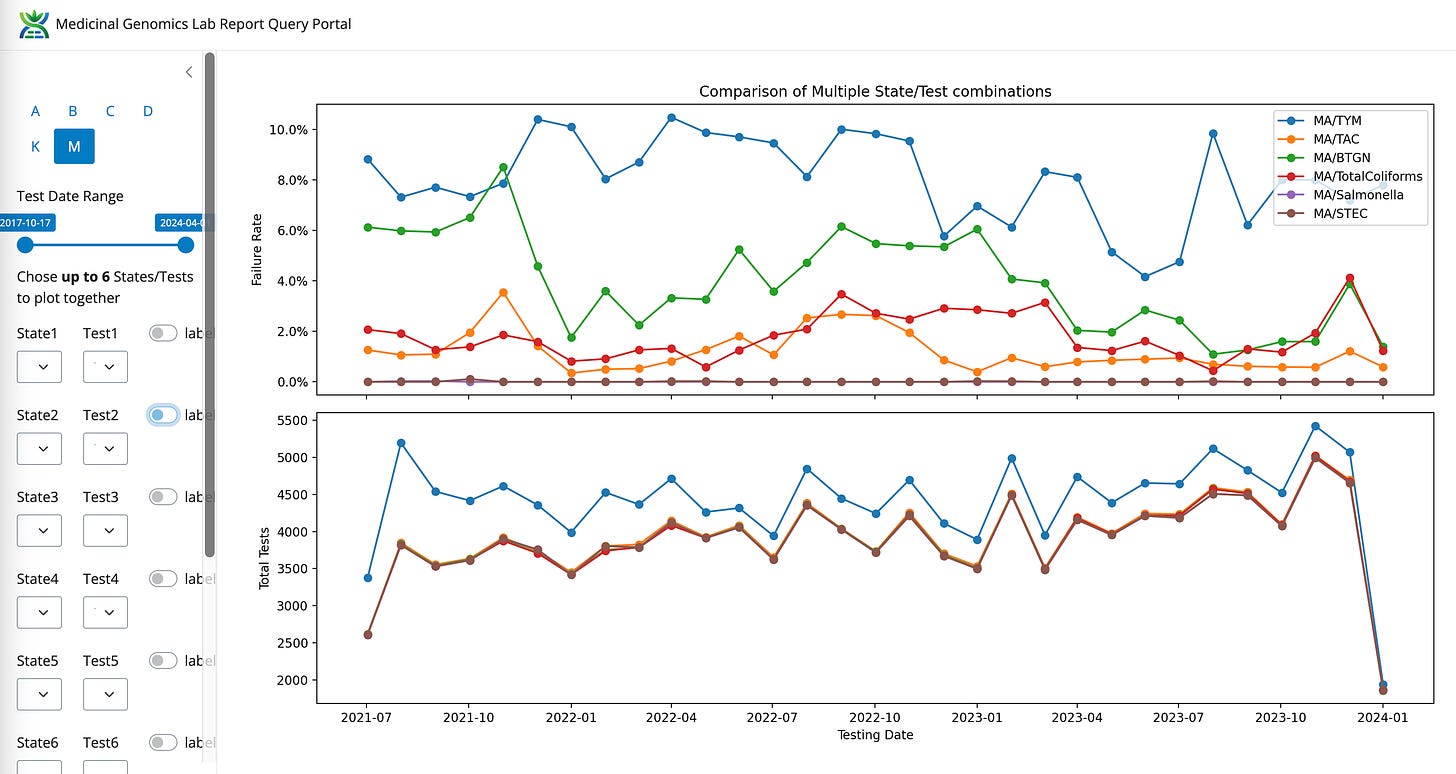
Longitudinal data.
All Mass Microbial Test failure rate over time
E.coli and Salmonella tests have such a low failure rate that these tests shouldn’t induce any lab shopping. Lets zero in on the other 4 tests and see if there are any trends.
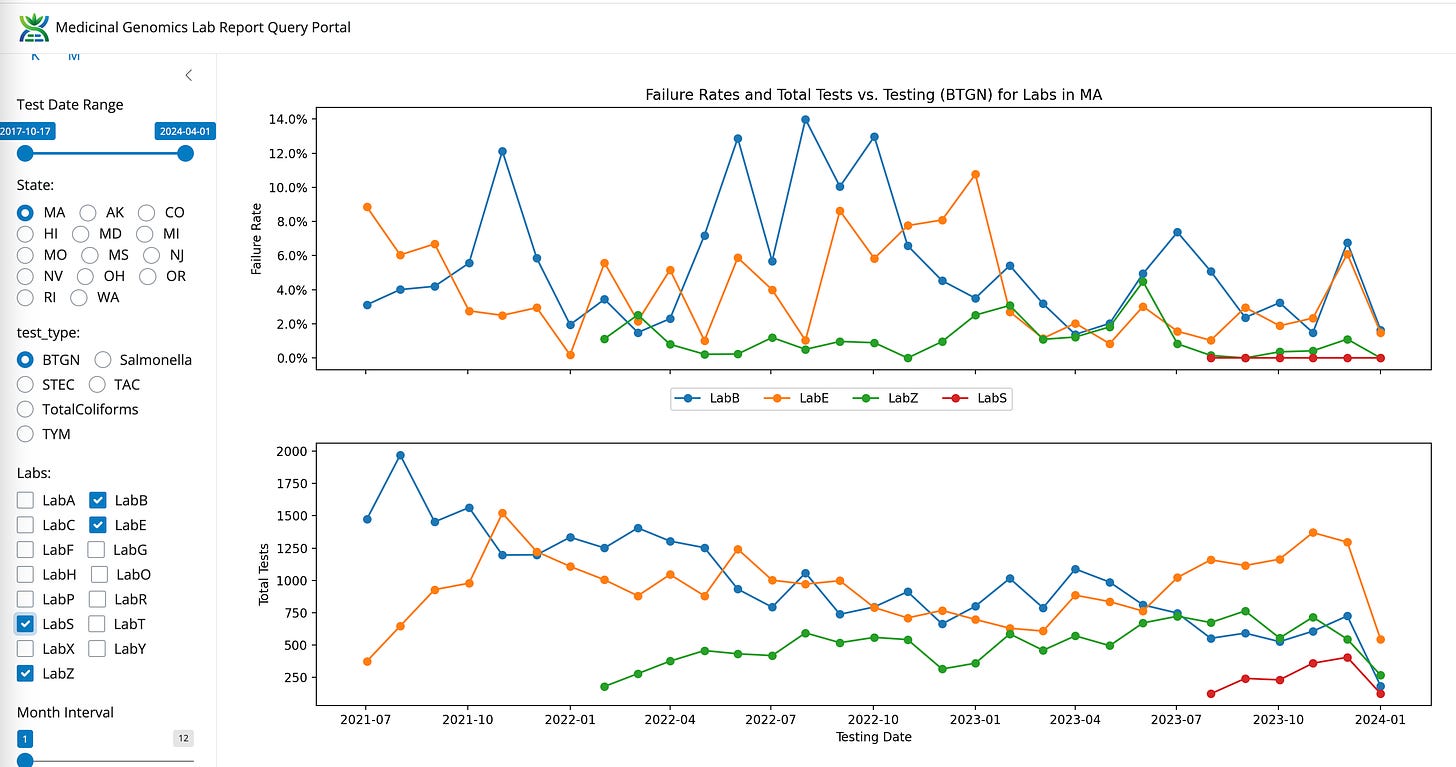
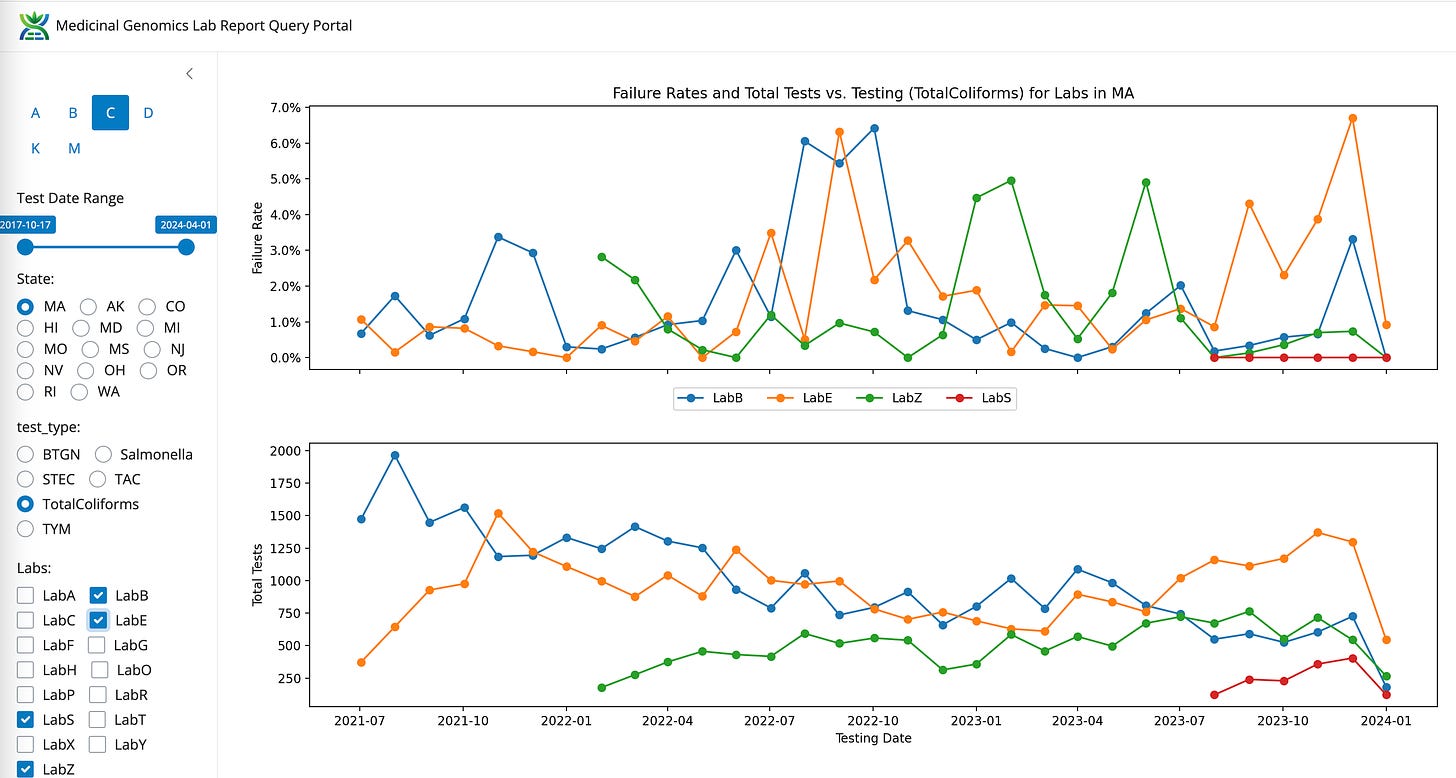
Coliforms between LabB (qPCR and Plating), LabE (qPCR) and LabZ (End Point PCR)
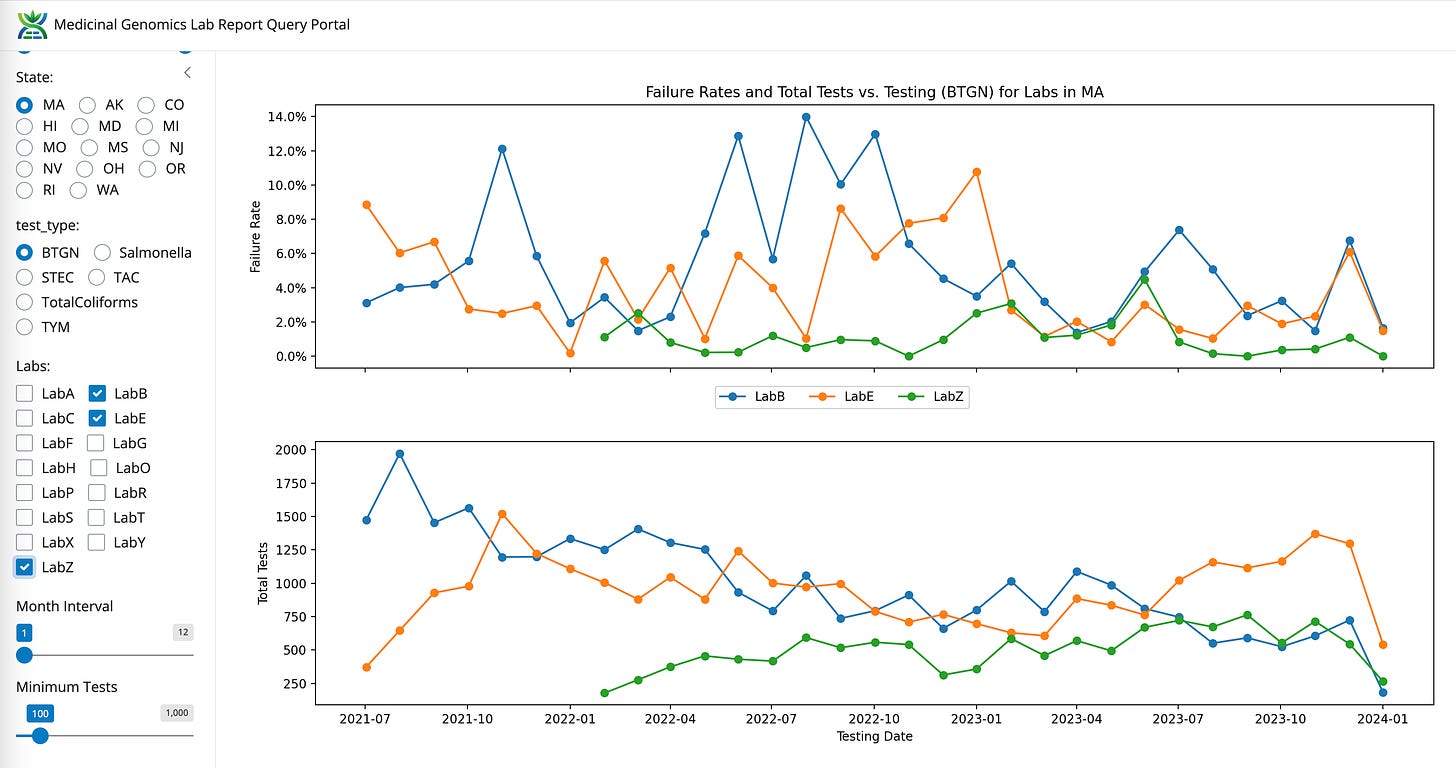
BTGN between LabB, LabE, and LabZ
TAC for LabB, LabE, LabZ
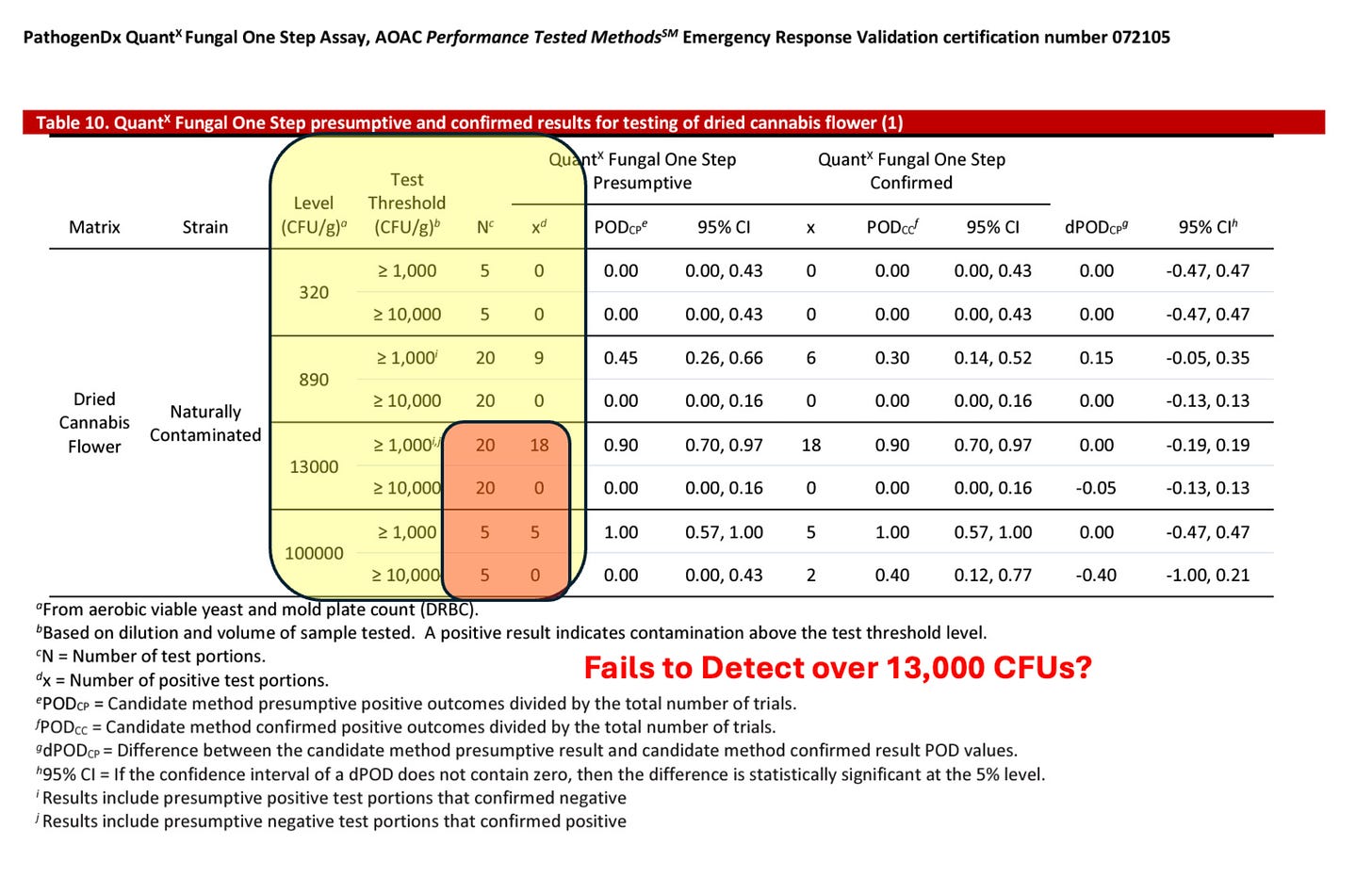
Recently, a new lab entered the Massachusetts market (LabS) with former CDx employees using an End Point PCR system. This platform has AOAC certification documents on TYM testing that suggests the platform can not detect over 13,000 CFUs/g.
LabS has 10X lower TYM failures than even LabZ. Both are using the same platform and both have some of the lowest microbial failure rates in the state.
Volumes are climbing for LabS and LabZ. Volumes are decreasing for LabB and appear neutral for LabE.
LabS also has the lowest BTGN failure rate (Red).
LabS can’t seem to detect any Coliform! Zero fail rate in the state (Red).
Summary of all Tests. LabS and LabZ are outliers and using the same End Point platform.
Why does this End Point PCR platform pass so many samples?
If you look at their AOAC TYM certification, the platform cannot detect over 13,000 CFUs. This implies the platform is saturating. Likely overloaded DNA preparations as this artifact goes away with their automated system that uses a different prep. Samples with very high CFU fail to trigger their 10,000 CFU/g threshold but do trigger their 1,000 CFU/g threshold (see orange box). The most heavily contaminated samples are passing.
This results in TYM data that cannot be ignored. All the Yeast and Mold in the state got the memo to never grow above 10,000 CFUs/g.
Compared to LabB and this LabZ’s data looks entirely un-natural.
AOAC doesn’t have SMPRs for TAC, BTGN or Coliform tests in Cannabis so there is no AOAC approval process for these tests unless you already have these tests AOAC approved in the food industry and perform a matrix extension study in Cannabis.
This AOAC policy favors incumbent parties and squeezes out the smaller assay providers that are not in food testing. Massachusetts doesn’t have AOAC requirements.
It doesn’t appear AOAC certifications would rescue the state as AOAC certified platforms that can’t detect TYM over the 10K action limit are actually the cause of the problem in the state. They are currently used in Massachusetts despite the certification demonstrating the platform is not fit for purpose.
For the grows using these labs, you should know that all of this data is public now. One can easily track grows hopping from one lab to another looking for lower failure rates in the CCC released data. If a patient gets injured and they can track the sample back to your grow, all their attorneys will need to do is look you up in these public data to see if you lab shopped for lower fail rates. Its clear this is happening in the data.
The public data has a comment field that identifies the labs. We have refrained from explicitly naming them as we are not certain the CCC meant to include that data given they made attempts to cipher the Metrc barcodes.
How do we fix this?
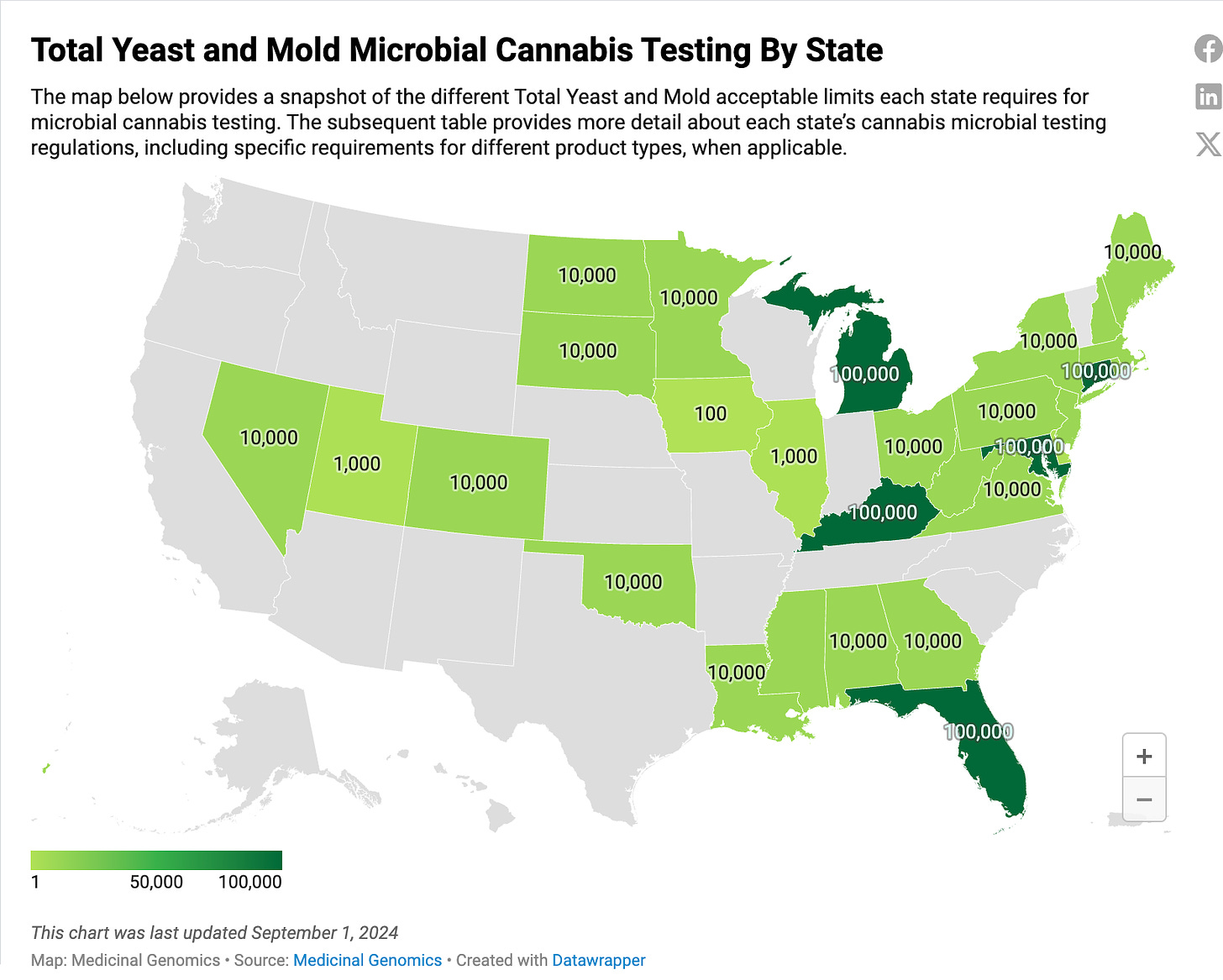
Massachusetts cannabis markets are not in a vacuum. Both CT and NY have raised their TYM limits to 100,000 CFUs/g and instituted single CFU Aspergillus testing to target the labs on quantifiable clinical risks. In states that have implemented 100,000 CFUs/g like MD and MI, the TYM failure rates drop to ~3% and the labs with the higher fail rates retain the largest market share. This is a sign that at these thresholds, microbial lab shopping pressures are eliminated. In states like NV and MI, Aspergillus testing creates 2-3% fail rates. In 100% indoor grown Alaska, Aspergillus failures are under 1%.
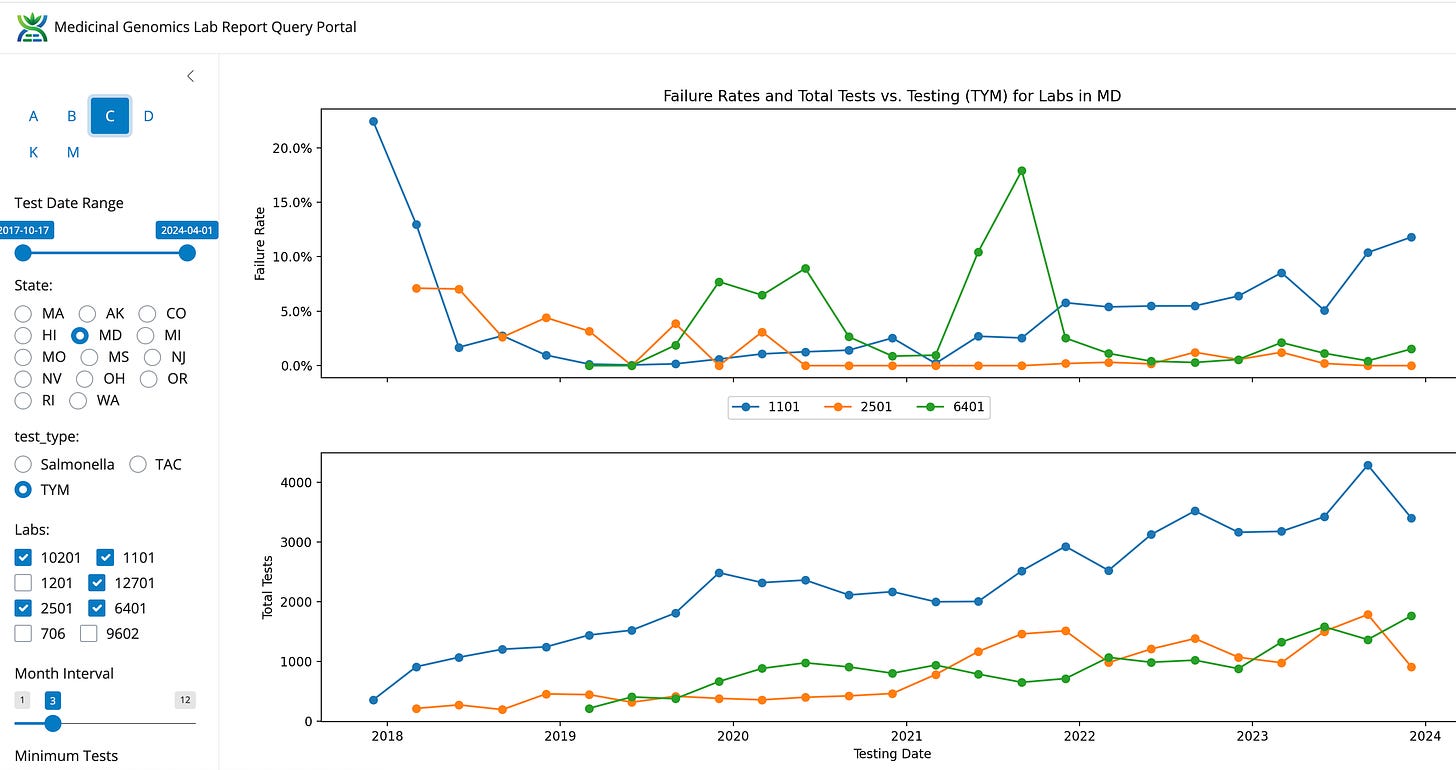
A side note- Lab 1101 is owned and operated by the same team as LabE in Massachusetts. They have the highest TYM failure rate and the most market share in MD.
Economic argument
If Massachusetts continues with TYM testing that induces 8% (0.24%-13.3%) failure rates, prices will rise compared to CT and NY, lab shopping will accelerate towards the 0.24% failure rate LabS and faith in the testing program will continue to erode. Higher prices will result in Massachusetts customers shopping in neighboring states.
Clinical argument
There are ample cases of Cannabis induced Aspergillosis in the clinical literature. We cannot find clinical literature for 10,000 CFU/g of TYM vs 100,000 CFU/g of TYM. There is reason for this dearth of data. Clinical sciences require you perform Koch’s postulates to draw associations with pathogens and clinical risk. This requires you isolate a pathogen and reintroduce it to a host and demonstrate recapitulation of disease. As a result of this, all of the clinical literature is anchored in species specific pathogens. Since you cannot perform Koch’s postulates on a population of microbes (TYM or TAC), there is no clinical literature to support the test. Relaxing the test that has the least clinical support and induces the most lab shopping can be clinically justified if stringent testing is put in place for actual pathogens with documented clinical risk like Aspergillus.
Legal argument.
Labs have been raising the lab shopping problem in Massachusetts for nearly 2 years now. No enforcement has transpired. Additionally, the CCC is currently in a state of reorganization suggesting enforcement actions against low fail rate labs is unlikely to occur in a time frame that will salvage honest labs. Considering enforcement actions in other states like California, Oregon and Michigan have all turned litigious, I would not be holding my breath for an organization in disarray to embark on potentially litigious enforcement action against labs with low failure rates.
If you are an honest lab, your best chance of survival is to change the regulations to match those of neighboring states. This will minimize cross state competition from grows with more lenient regulations. In Massachusetts, we must remain aware of most customers being only a 90 minute drive from more clinically rational and more lenient regulation.
Grows have more cash flow than labs. If Massachusetts continues with TYM testing that is not clinically defendable in court, grows will take them to court and remove the testing as has happened in Oregon.
A lesson from Oregon
As Oregon attempted to implement Aspergillus testing, many labs burning in these new protocols had indefensibly high Aspergillus failure rates (23-100%) that did not mirror the Aspergillus fail rates seen in other states like MI and NV. This was likely an artifact of labs not having enough time to burn in new tests. As a result, grows panicked and sued the state. This was entirely avoidable if the state had their hands on comparative data from other states to help identify the labs that were out of spec before allowing out of spec labs from failing product and creating panic in the marketplace.
Based on these arguments, we highly advise the CCC to consider a rule change that harmonizes the Massachusetts microbial regulations to mirror those of CT and NY.
100,000 CFU/g for TYM
Institute Aspergillus testing at Less than 1 CFU/g
There is an important distinction between zero CFU/g and less than 1 CFU/g.
Less than 1 CFU/g scales to less than 10 CFU per 10 grams where as Zero does not.
10 gram testing is commonly used in the food industry and as cannabis prices come down it may get implemented in the cannabis space to solve the sampling issues seen with testing 1g from 50 pounds (1gram out of 22,679 grams).
This rule change should cut the TYM failure rate from 8-12% range down to 2-3% while inducing a 2-3% Aspergillus failure rate. This change will remove the largest lab shopping pressure in the state related to microbial testing and provide more clinically defensible testing program in line with neighboring markets.
To address THC lab shopping we recommend monthly publication of testing data so THC inflation can be publicly tracked and bad actors exposed.
If you are a grow or a lab that supports these changes and wish to be a signatory of a letter petitioning for this rule change. Please contact us at info[at]medicinalgenomics.com
Testing regulations by State. Note NY recreational markets are not reflected on this map. The recreational TYM test is not a pass fail test in NY. Its merely for monitoring.
Disclaimer- We manufacture qPCR kits sold into this market. We have a vested interest in an honest market. The data delivered directly from the CCC is linked in the top of the article. If you would like access to the Web Browser we built to view these data, please contact us info[at]medicinalgenomics.com




Hello Mr. McKernan.
I've been very concerned with the DNA contamination of the Covid mRNA vaccine. I want to ask for your opinion on the new GMO vaccine for dengue: Qdenga.
Jan 2024, Brazil began vaccinating its population with Qdenga. Now, the total number of cases rised 3.5 fold, fatality 5 fold, CFR 1.38 fold.
https://www3.paho.org/data/index.php/en/mnu-topics/indicadores-dengue-en/dengue-nacional-en/252-dengue-pais-ano-en.html
Qdenga can cause viremia, and it's a GMO product.
I'm really concerned about this new vaccinating campaign, but I'm no expert, just a reader. So, I seek for your opinion on this: can this GMO product cause another large scale disaster like the Covid mRNA jabs?
This is quite interesting from the perspective of an Australian user. The TGA is once again taking the money for minimal work. They must have the best union in the universe.
<<Therapeutic goods supplied in Australia must comply with applicable standards and other requirements. For most medicines these are 'default standards' provided by the British Pharmacopoeia, European Pharmacopoeia, and United States Pharmacopeia - National Formulary.>>
And
<<Results of TGA Laboratory testing have historically been released every 6 months in the Database of TGA laboratory testing results. However this database has temporarily been removed due to technical difficulties. We are working to get the database uploaded again soon.>>
And I can only find one attempt to perform any work mentioned in your article from 2021
<<Testing for toxins in medicinal cannabis products being supplied via SAS B in Australia
26 August 2021 Report on the laboratory testing for toxins in medicinal cannabis products being supplied via SAS B in Australia>>