One of the reasons we believe DNaseI is failing to fully digest the DNA in the modRNA vaccines is because the IVT reaction that makes modRNA from dsDNA templates (plasmids) creates molecules that are DNA:RNA hybrids.
This has DNA on one strand and RNA on the other. DNaseI doesnt recognize these molecules and when you have a vast excess of RNA that is complementary to a DNA template the DNA will base pair with the RNA instead of its complement DNA strand which is in much lower quantity than the RNA.
We covered this in a former substack but until now it was all hypothetical.
One of the work horses of genomics is an enzyme known as RNaseH which stands for RNase Hybrids. This is an enzyme commonly used to make cDNA libraries as it will erase the RNA once cDNA is synthesized from an RT polymerase.
Since we frequently make RNA-Seq libraries for sequencing Hop Latent viroid infected cannabis we happened to have some of this enzyme in the freezer and I simply applied it to vaccine mRNA while exploring its impact on purely circular HpLVd RNA (Hop Latent Viroid).
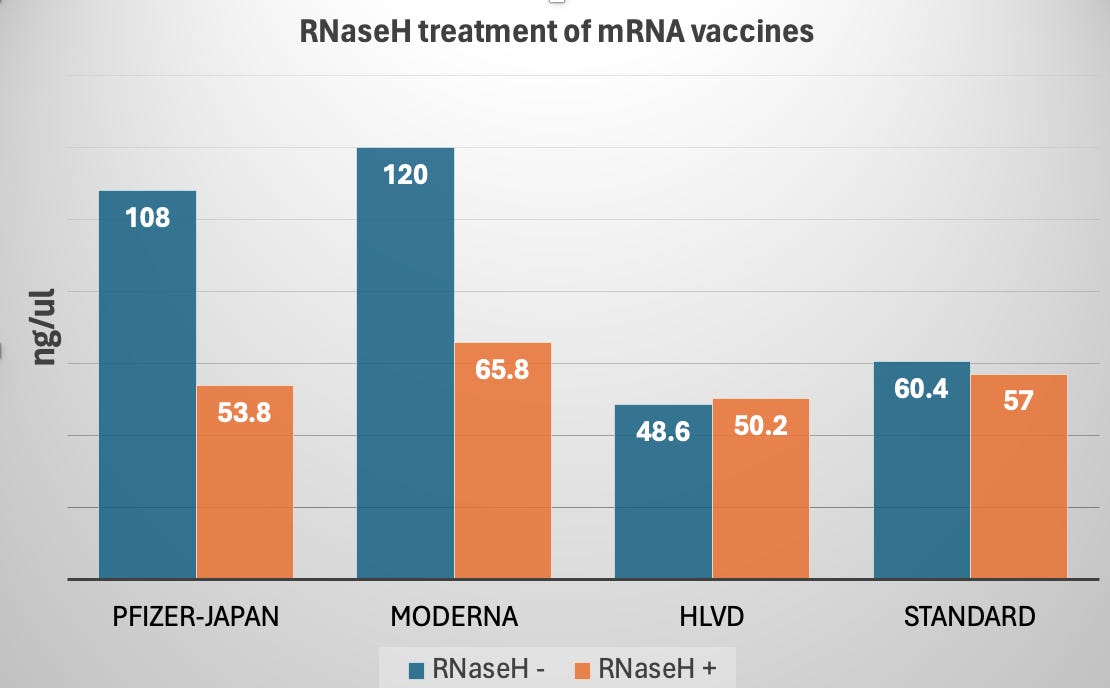
In this experiment we are using a ssRNA specific dye known as AccuBlue from Biotium. This is a similar dye to RiboGreen from Invitrogen. We can see 108-120 ng/ul of RNA in the vaccines before a 10 minute treatment with 2ul this enzyme. Once treated the RNA signal drops ~50%.
When this enyzme is used on pure circular RNA (HLVD) and the RNA standards from the AccuBlue quantitation kit we see no material change in the amount of RNA present. This implies a lot of the RNA in the vaccines is in fact DNA-RNA hybrids which is resistance to DNaseI digestion.
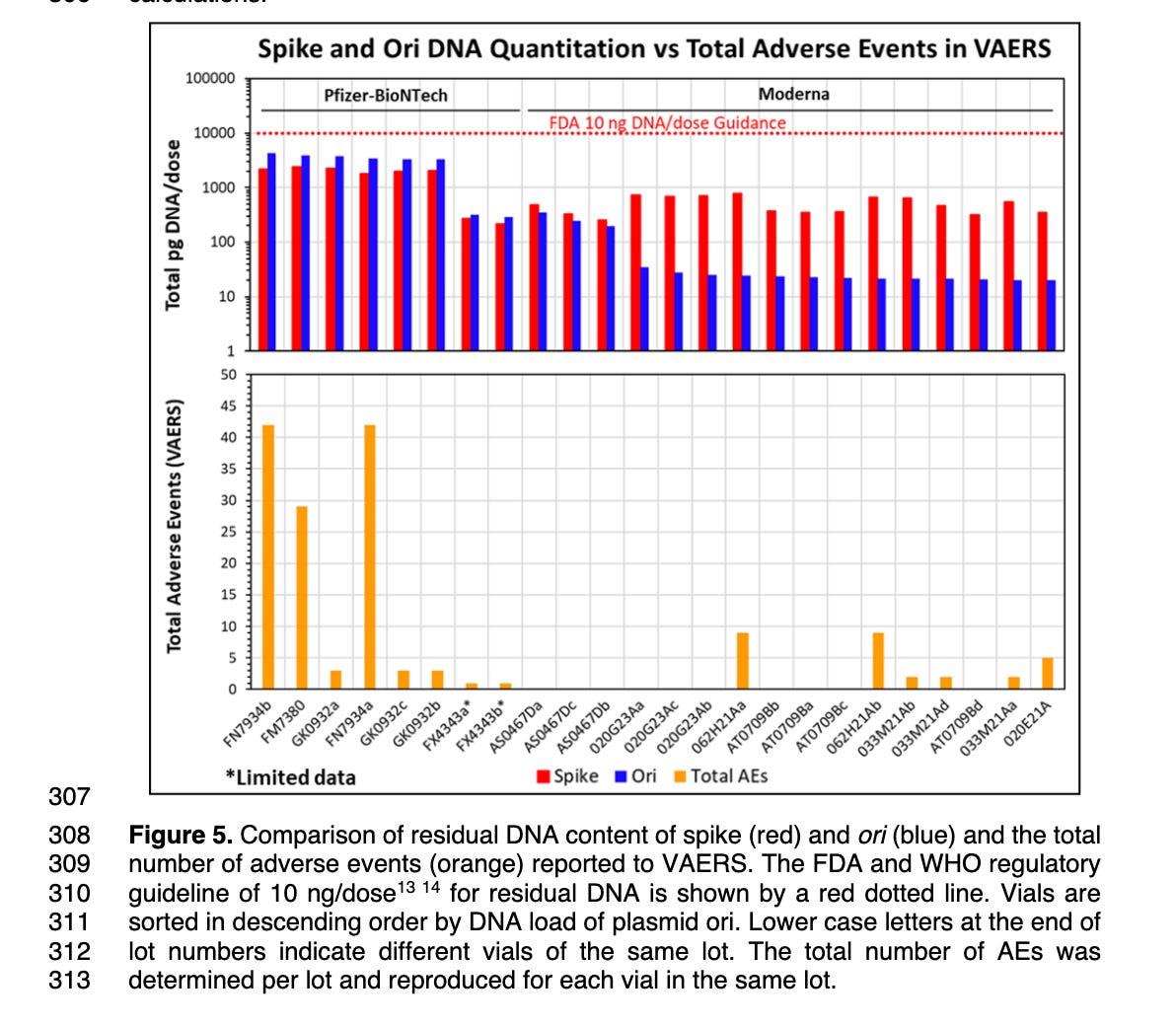
This likely explains why many of the Moderna lots have very different amounts of Spike DNA contamination compared to Vector DNA. This is seen in Speicher et al Figure 5 where the red lines in many Moderna lots have many orders of magnitude more Spike DNA (Red) compared to Vector DNA (Blue) as assessed by qPCR. Their DNaseI conditions are doing a better job of eliminating the DNA that has no RNA complement. Not all of the Moderna lots exhibit this behavior and none of the Pfizer lots exhibit this behavior.
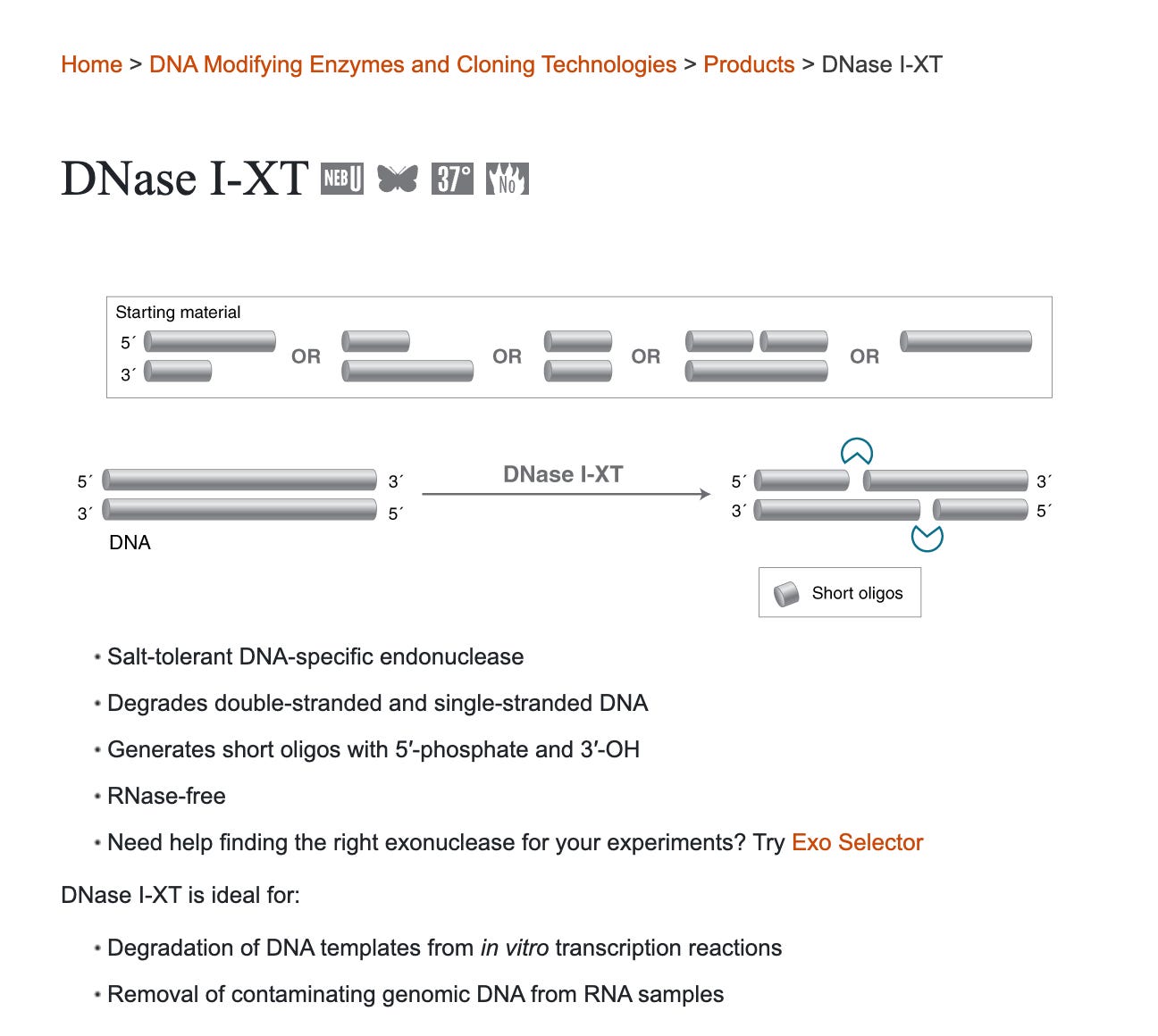
There are some DNases on the market that claim to do a better job digesting DNA in DNA:RNA hybrids.
One is known as DNaseI-XT from NEB
Its possible that Moderna and Pfizer are using different DNases in attempt to do a better job eliminating the DNA contamination and this is why we see differences in Spike/Vector DNA contamination in some lots.
Conclusions
Not only is there dsDNA contamination that exceeds the EMA and FDA limits in the modRNA vaccines, but we now know there is dsRNA contamination and RNA-DNA hybrids. What happens when this soup of contaminants is transfected into a cell is anyones guess.
We already know from the Kwon paper that cytosolic DNA transfections can trigger cGAS-STING and lead to oncogenesis.
dsRNA transfections will trigger the RNAi pathway.
DNA:RNA hybrid transfections are less studied.
Add in the complexity of the N1-methyl-Pseudo U modRNA and all the frame shifting it induces, and we have real bathtub gin masquerading as precision medicine.
We can only hope this doesn’t leave us Down with Disease…
with a fully tweaked Golgi Apparatus
Im off to the Sphere this weekend. First time in that venue. Wish me luck.





thank you Kevin, again
I plugged some questions into ChatGPT ..
Question:
What negative pathologies can be triggered in humans from double stranded RNA?
Answer:
Double-stranded RNA (dsRNA) can trigger several negative pathologies in humans due to its role in the immune response and its association with viral infections. Here are some key negative effects:
Immune Activation and Inflammation:
Innate Immune Response: dsRNA is recognized by the immune system as a marker of viral infection. This recognition occurs through receptors like Toll-like receptor 3 (TLR3), retinoic acid-inducible gene I (RIG-I), and melanoma differentiation-associated protein 5 (MDA5). Activation of these receptors leads to the production of interferons and other pro-inflammatory cytokines, causing an inflammatory response .
Chronic Inflammation: Prolonged or excessive activation of the immune system by dsRNA can lead to chronic inflammation, contributing to the development of inflammatory diseases .
Cellular Stress and Apoptosis:
Endoplasmic Reticulum (ER) Stress: dsRNA can induce stress in the endoplasmic reticulum, leading to the unfolded protein response (UPR). If this stress is unresolved, it can result in cell apoptosis (programmed cell death) .
Direct Induction of Apoptosis: The immune response to dsRNA can directly induce apoptosis through pathways involving caspases and other apoptotic mediators .
Autoimmune Diseases:
Molecular Mimicry: dsRNA can sometimes lead to autoimmune diseases through molecular mimicry, where the immune system targets host tissues that resemble viral components. This can contribute to diseases such as systemic lupus erythematosus (SLE) (Mark Dreyfus).
Cancer:
Chronic Inflammation and Cancer: Chronic inflammation induced by persistent dsRNA can contribute to the development of certain cancers. Inflammation is a known risk factor for cancer, and the pro-inflammatory environment can promote mutations and other cancerous changes in cells (Law Society Journal).
Genomic Instability: Persistent dsRNA can also contribute to genomic instability, which is a hallmark of cancer development. This instability can arise from the chronic activation of DNA damage responses and other stress pathways (Women's Agenda).
Neurological Disorders:
Neuroinflammation: dsRNA-induced inflammation can affect the central nervous system, leading to neuroinflammation. This can contribute to the pathology of neurological disorders such as multiple sclerosis (MS) and Alzheimer's disease (The Lawyer Magazine).
Direct Neuronal Damage: In some cases, the immune response to dsRNA can lead to direct damage to neurons, exacerbating neurological conditions .
These pathologies illustrate the complex and potentially harmful effects of dsRNA in the human body, especially when it triggers an inappropriate or excessive immune response.
References
Kawai, T., & Akira, S. (2006). Innate immune recognition of viral infection. Nature Immunology, 7(2), 131-137.
Alexopoulou, L., Holt, A. C., Medzhitov, R., & Flavell, R. A. (2001). Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature, 413(6857), 732-738.
He, B. (2006). Viruses, endoplasmic reticulum stress, and interferon responses. Cell Death and Differentiation, 13(3), 393-403.
Zamanian-Daryoush, M., & Williams, B. R. (2006). Functional implications of protein kinase R in the interferon response. Journal of Interferon & Cytokine Research, 26(12), 877-889.
Gerloni, M., & Zanetti, M. (2005). CD4 T cells in autoimmune diseases: a role for self/nonself recognition and Th1/Th2 switch. Springer Seminars in Immunopathology, 26(4), 359-376.
Grivennikov, S. I., Greten, F. R., & Karin, M. (2010). Immunity, inflammation, and cancer. Cell, 140(6), 883-899.
Jackson, S. P., & Bartek, J. (2009). The DNA-damage response in human biology and disease. Nature, 461(7267), 1071-1078.
Heneka, M. T., Golenbock, D. T., & Latz, E. (2015). Innate immunity in Alzheimer's disease. Nature Immunology, 16(3), 229-236.
Ransohoff, R. M., & Brown, M. A. (2012). Innate immunity in the central nervous system. Journal of Clinical Investigation, 122(4), 1164-1171.
.. when trying to ask questions about DNA:RNA hybrids .. ChatGPT doesn't seem to want to discuss the topic and chucks a fit .. telling
Thank you Dr. McKernan for helping to confirm the hypothesis, although in many ways I'd rather it wasn't!