On June 15th, 2023 I presented to the FDA on the dsDNA contamination we found in the Pfizer and Moderna vaccines.
A preprint on this discovery can be found on OSF.io.
This DNA contamination is important as it is a result of a manufacturing change Pfizer performed after the clinical trial. This is evident in the EMA documents and published in the BMJ (Levi and Guetzkow). Process 1 used synthetic DNA for the trial. Process 2 was used to scale up the manufacturing and this required cloning of the vaccine encoded DNA into a plasmid for replication in E.coli. This process is materially different but was treated as a bio-equivalent.
A small 250 person test was performed comparing Process 1 to Process 2 and the results demonstrate higher adverse events in the Process 2 material and lower RNA integrity (Levi and Guetzkow).
A recent analysis of Pfizer's pivotal study (Levi and Guetzkow) found 1.5-3 fold higher rates of adverse events in patients after crossover and presumably receiving Process 2 material, compared with patients before crossover, presumably receiving Process 1 material.
From Levi and Guetzkow-
"The protocol amendment states that “each lot of ‘Process 2’-manufactured BNT162b2 would be administered to approximately 250 participants 16 to 55 years of age” with comparative immunogenicity and safety analyses conducted with 250 randomly selected ‘Process 1’ batch recipients. To the best of our knowledge, there is no publicly available report on this comparison of ‘Process 1’ versus ‘Process 2’ doses."
The Vaccine Efficacy and safety was broadcasted to the world based on the Process 1 results. But the Process 2 vaccines are now known to have plasmid derived double stranded DNA in the vials. This contaminant was not part of the informed consent process nor was it present for the Process 1 RCT.
We have summarized our work in the following preprint.
Using multiple analytical methods we determined the dsDNA contamination was 18-70 fold over the 330ng/mg DNA/RNA guideline set by the EMA. It is also over the 10ng/dose guideline by the FDA. Citations for the guidelines are listed in the Preprint.
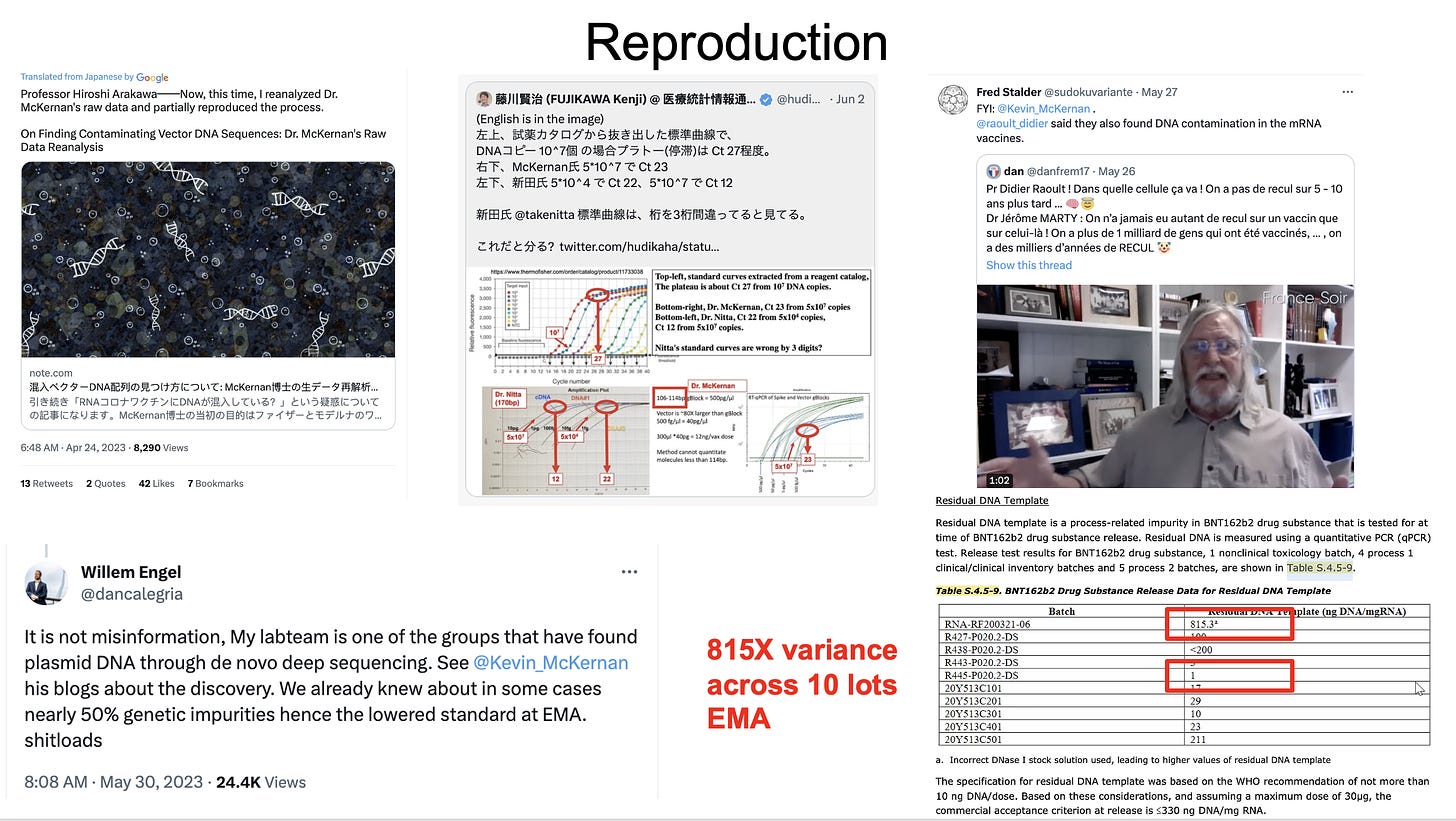
PrePrints are not peer reviewed but the best peer review is actual wet lab reproduction of the work by independent scientists. Several scientists from around the world have replicated parts of this work as disclosed in the FDA VRBPAC meeting.
First, the EMA noted an 815 fold variance in dsDNA contamination in the 10 vials Pfizer provided data for. The process for removing this dsDNA was noted as being variable and poorly understood by the EMA.
Recommendations by the EMA
“The Marketing Authority Holder (ie Pfizer/BioNTech) should reassess the specification for the linear DNA template purity and impurities.
The Applicant has already agreed to supply these by Q2 2021.
The MAH should provide the results of the studies performed to enhance the robustness of the DNase digestion step.
The MAH should comprehensively describe the capability of the next generation sequencing technology platform to detect lower amounts of RNA species of alternative sequence in the presence of the correct, more abundant RNA for the active substance.”
Secondly, a group in Europe (Willem Engel) produced Illumina sequencing of BNT162b2 and found the same plasmid sequence we identified.
Third, a group in Japan, downloaded our raw reads and performed their own assembly and analysis and also detected the Pfizer plasmid.
Forth, Didier Raoults lab mentioned they also found DNA in the vaccines but not data has been published to date.
Since the FDA meeting, Dr. Sin Lee of Milford Molecular diagnostics took our published primer sequences and amplified BNT162b2 and Sanger sequenced the amplicons to prove they were in fact plasmid derived dsDNA sequences.
The Pfizer plasmid maps disclosed to the EMA omitted key aspects of the plasmid known as the SV40 promoter, SV40 Enhancer, The SV40 Origin of replication and the SV40 polyA signal. This is 466 bases of the vector that is derived from the SV40 virus. It does not contain the full 5,243 base pair SV40 virus but some of the key elements for hyper expressing genes are present in the Pfizer monovalent and bivalent vaccines.
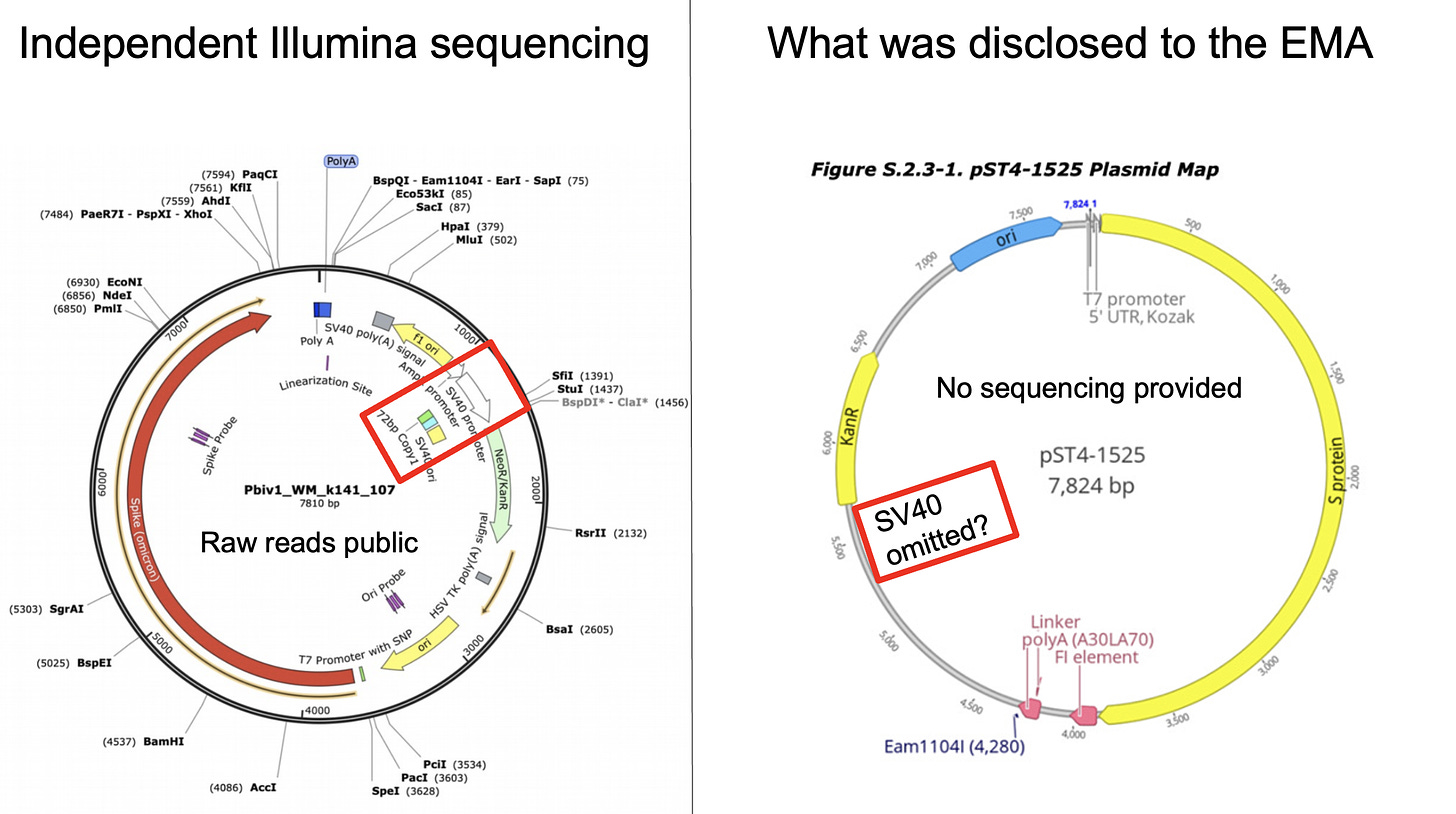
SV40 virus is a controversial sequence as it contaminated the polio vaccines and is still debated to this day if it caused 100 million cancers. While the vaccine does not contain the full virus sequence, 2-20% of the population is believed to be SV40 infected in part due to the polio vaccination program. It is not known what will happen if we inject SV40 infected patients with large quantities of SV40 promoters, enhancers or origins of replication.
These SV40 promoters contain a strong nuclear localization signal known as the 72bp SV40 enhancer. Dean et al describes this nuclear location of the dsDNA SV40 enhancer contamination now known to be in the Pfizer vaccines. If any of these plasmids remain intact, Baiker et al demonstrate plasmids with the SV40 origin of replication can replicate for 100 cell generations as an episomal (non integrated but nuclear persistent) sequence.
These nuclear integration risks are not hypothetical. They are the reason the FDA has these limits as described by Keith Pedens work. To further emphasize this point Strayer et al demonstrate even fragments of plasmid vectors can integrate into the genome with SV40 containing plasmids. Gonin and Gailard discuss the biodistribution risks of various gene therapy vectors. Banoun et al. describes why these mRNA vaccines qualify as gene therapy.
These contaminants are over the limit. Multiple independent scientists are reproducing these results. The injection of dsDNA containing controversial DNA sequences known to integrate into the genome was not properly disclosed to regulators nor patients in the informed consent process.
I want to thank Dr. David Wiseman, Dr. Maria Gutcha, and Dr. Janci Lindsay for helpful comments and suggested references for this document.





HOLY CRAP you're AMAZING for this and all your due diligence!!! At least SOMEONE is helping to hold these ass-hats accountable in some way!!!!!!!!!
A very helpful article that makes some things likely more easily understood by those not familiar with the material.