How did Aspergillus go extinct in Hawaii?
Hawaii is an interesting state to review for cannabis testing data in that it has only one lab since late 2022/early 2023 so there is currently no Lab Shopping dynamics.
In such circumstances, the lab may choose a particular platform for microbial testing and you can get a clean view of how that platform performs.
In this case the lab is using an EndPoint PCR system that is marketed as not requiring Enrichment for Aspergillus testing. This is an outlier. Most molecular systems require enrichment because you cannot amplify your way out of Poisson statistics.
The manufacturers makes claims it can skip enrichment but all the data we see in the field shows this platform has the lowest detection rate and this skipping enrichment cuts safety corners to achieve fast TAT for the lab.
Enrichment serves 2 purposes.
1)Eliminate dead DNA and focus the test only on viable organisms
2)Overcome Poisson sampling issues that arise due to subsampling large volumes.
The process of Enrichment for live-dead purposes is described in this previous thread.
The Poisson sampling issues raised in point #2 are often lost on many people but they relate to the fact that molecular biology likes to work in microliter sized assays (10ul) and cannabis sampling occurs in milliliters (10ml).
You can’t physically fit 1 gram of Cannabis into a 100ul qPCR reaction. You are usually wetting the cannabis in 10ml of solution and only qPCRing ~10ul-100ul of it (1/100th).
This invites Poisson sampling into the picture. If the sample has 1 CFU/g and you are only sampling 1/100th of the sample in qPCR, what are the odds you are going to find it with such a small subsample?
To overcome this subsampling issue, the field will grow the sample to compensate for the 100 fold subsample. If the organism doubles every 2hr, you can grow it for 6.6 doublings (13.2hr) and compensate for the 100 fold subsampling.
So Enrichment is performed to compensate for the fact that you are only testing a subsample of the total product and you need the sensitivity to find 1CFU/g.
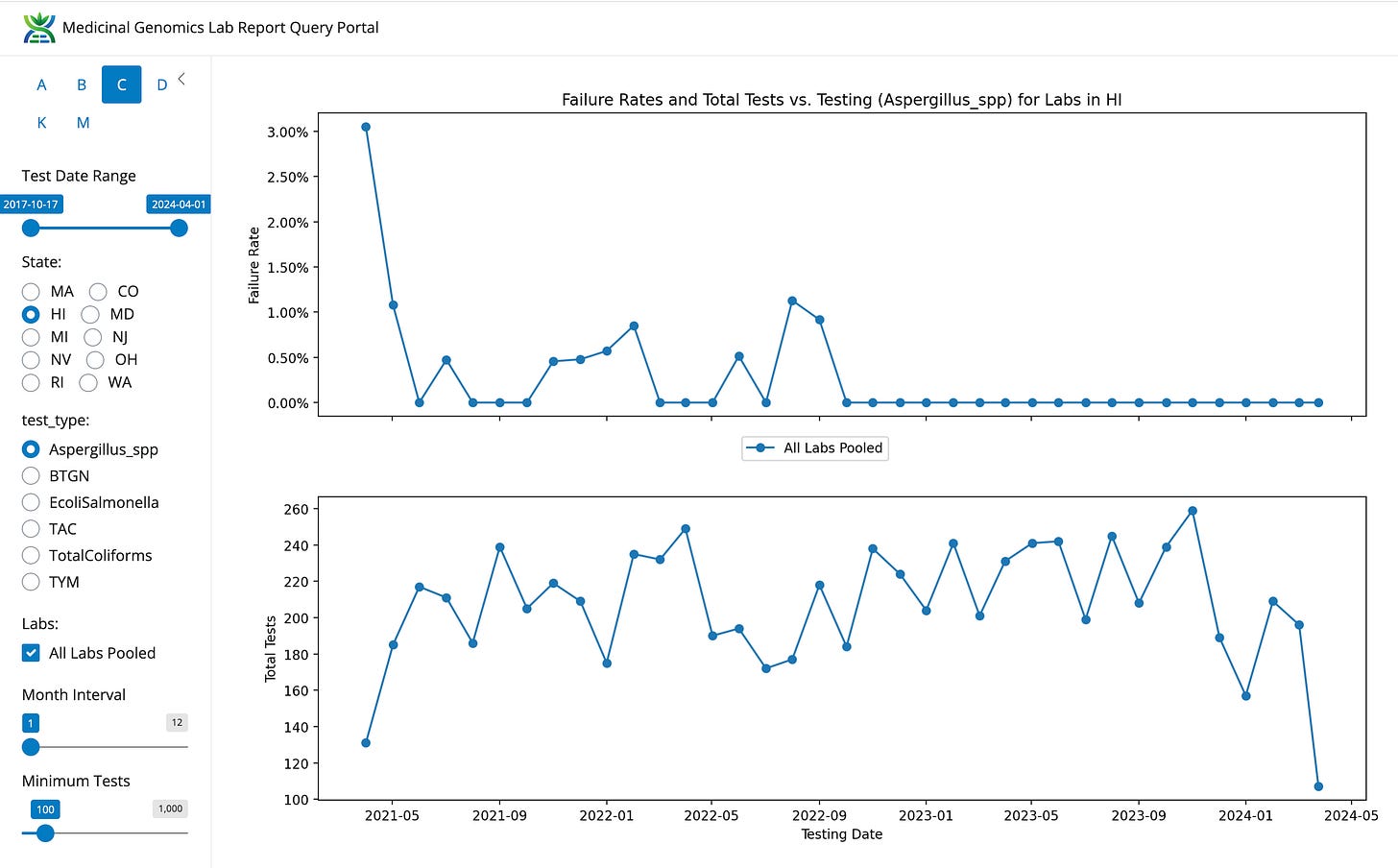
Hawaii state has the lowest Aspergillus failure rate we can find (0.72%).
Figure 1. Aspergillus failure rate but grow. Top line is the State average at 0.72%
Nevada is a 2.6% failure rate across all labs and has a very diverse install base using multiple different qPCR vendors.
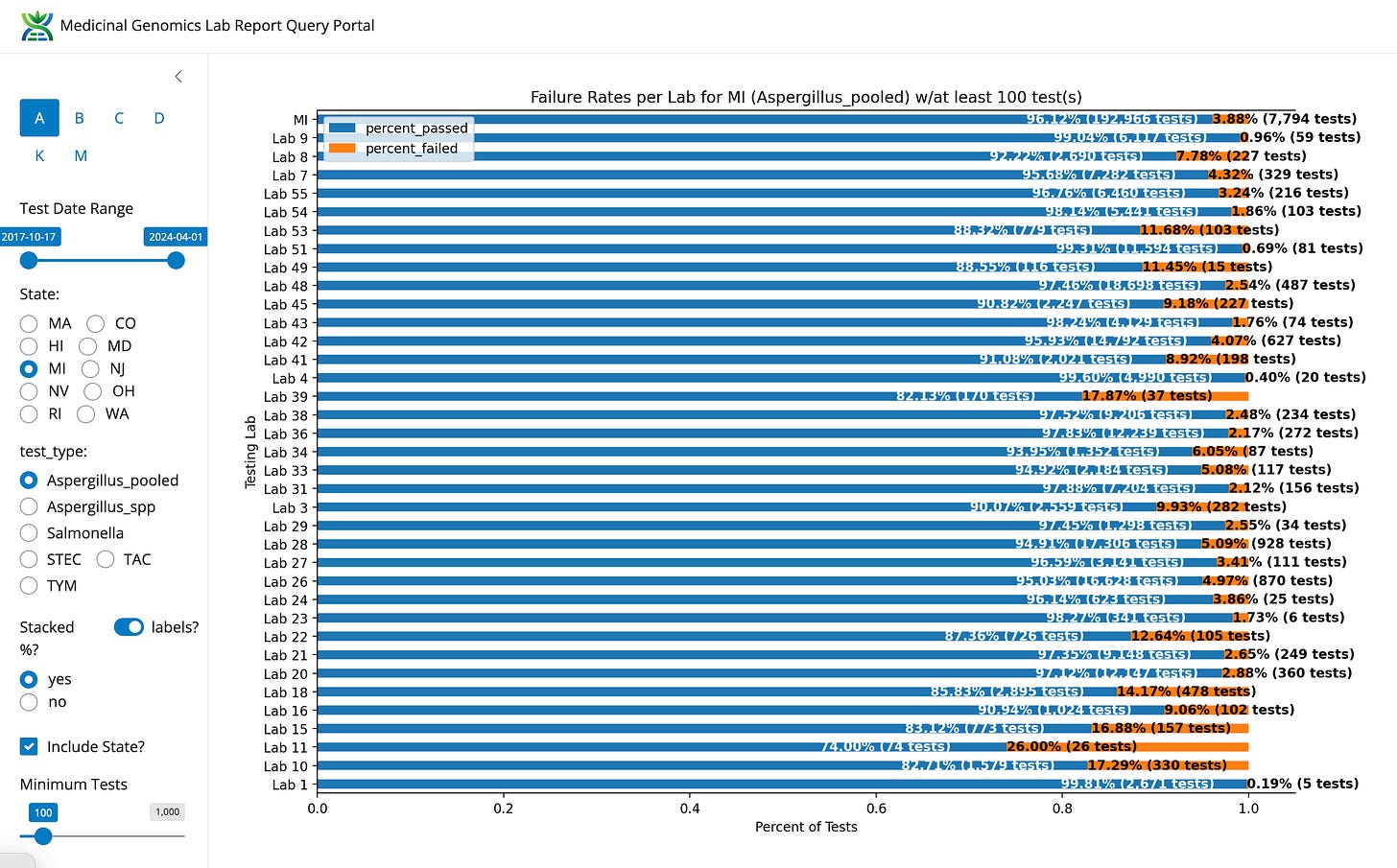
Michigan has similar diversity in tool providers and has a 3.88% Aspergillus failure rate.
Based on the Hawaii data, we can hypothesize which Labs in Michigan are running the enrichment free platform by sorting the labs on their Aspergillus failure rates.
Figure 2. Michigan Aspergillus failure rates by lab.
Figure 3. Aspergillus failure rates in Michigan, Nevada and Hawaii.
Prior to 2023, there were two testing labs in Hawaii. Every since there is only one lab using a platform that skip enrichment, Aspergillus has gone extinct in Hawaii while testing volume has maintained itself.
This may be a case of lab shopping driving honest labs out of business. The lab with the least sensitive test, owns the market.
Figure 4. Hawaii Aspergillus failure rate went to zero in late 2022? What happened?
The “State Lab Savior” boondoggle
This is also a reminder that the states can’t solve this complex regulatory problem by building a singular state lab that runs one vendors assay. If they do, how do we know its not like the scenario above?
This is an important point to drive home as most States are asking for more money to build labs as a proposed solution to this problem. These are costing tax payers millions of dollars and taking years to get online and certified. If they open their doors simply running one vendors assay, how are they different than just another lab in the state?
Are we to believe these people are made of finer clay? If the regulators use a single assay, they will drive all other labs in the state to run the same assay. This will create a regulatory capture attack surface on the market. Test providers will flock to gift, bribe, coerce and even defraud regulators into using their products knowing this dynamic will coerce all the labs to switch to the regulators test.
A better solution which can be implemented today for less money is transparent data and more secret shopper programs. Recalls need to have multiple vendor assays agree and multiple samplings confirm the event. Discordant samples require DNA Sequencing to ensure speciation of the PCR tests.
I hope state labs are funded as it will make for more informed regulators but in order for them to not play favorites in the market place, they will need to be very well funded so they can run every vendors method in the market place and commit to running multiple methods on each suggested recall.
I don’t think the market should be holding its breath on state labs suddenly fixing these problems when transparent data and secret shopper programs can be performed today for orders of magnitude less money. Both solutions may ultimately be required.
My state test trumps your market test
We do need to address the elephant in the room. in some states we are only sampling 1g out of 50 pounds of cannabis. or 1 gram out of 22,659 grams!
It would be insane to expect 100% concordance with the State Lab from a single testing point. They will need to run testing at least 10 times to have confidence in a recall.
The food industry uses 10-25 gram samplings and AOAC will not certify any cannabis test at 1g because they understand these sampling statistics are insane. It will create chaos in the field if the regulators don’t recognize that proof of a recall must rest on high reproducibility findings with multiple assays.
Test vendors will be quick to blame each others assays for failure when the majority of the time the discordance will simply be a matter of sampling statistics.
In the case of Hawaii and Aspergillus going extinct… That looks like a clear case of a test never finding anything and it should be assessed with assays with known detection rates in other states.
This is the most important health risk in Cannabis. If you are not going to test for Aspergillus, there is no point testing for anything else. Nothing else has the death rate in Cannabis. Try to find papers on deaths associated with any other Cannabis testing analyte. It won’t come close to Aspergillus and its economic impact.
Economics of Aspergillosis.
Microbial tests are $10-$100 where 1g is usually tested from a 50 pound batch.
If you assume $900/pound, This is $100 cost for $45,000 batch or 0.22% increase in cost per batch.
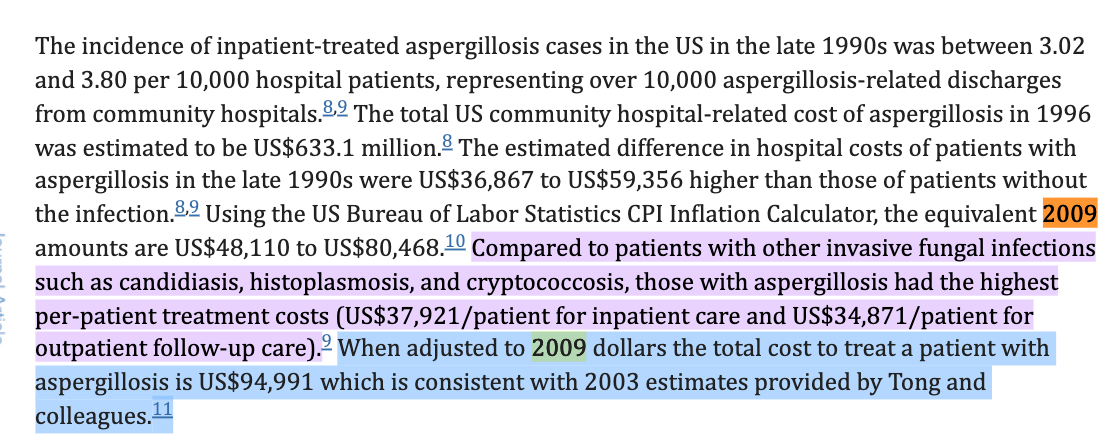
Aspergillosis is estimated to cost ~$94K to treat in 2009.
This is close to $1B annual cost.
Other more recent CDC estimates are 15,000 hospitalization per year at $1.2B in 2014.
“The number of hospitalizations related to invasive aspergillosis in the United States increased an average of 3% per year during 2000-2013. Nearly 15,000 aspergillosis-associated hospitalizations occurred in the United States in 2014, at an estimated cost of $1.2 billion.”
These costs have likely risen with inflation 10 years later.
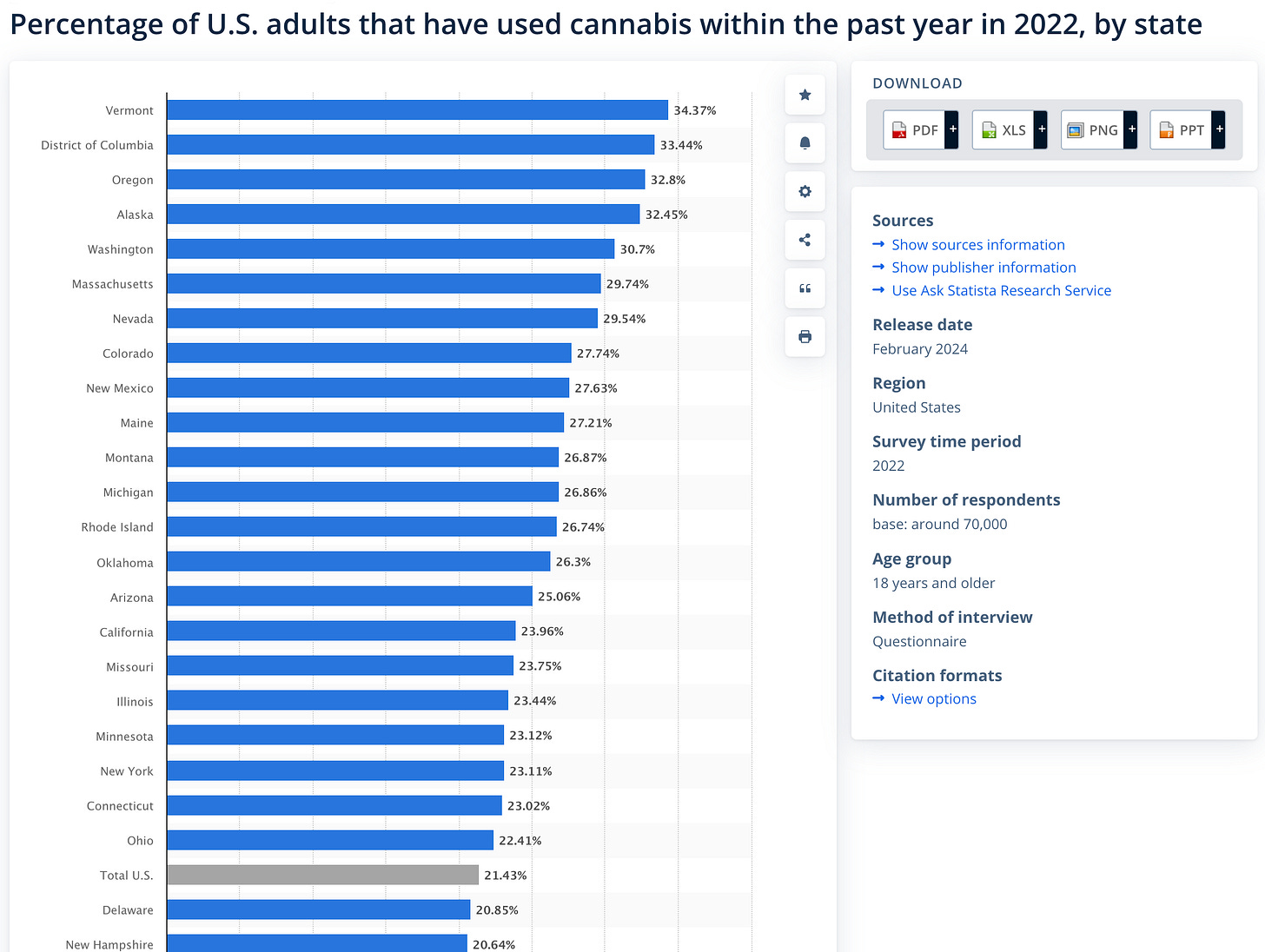
Another CDC study of ICD10 codes shows Cannabis users have a 3.5X increase in fungal infections. 21% of the US population are cannabis users with some state estimates in OR and VT ranging as high as 32-34%
If we ignore the CDC 3.5X enrichment, and just take the US cannabis use rate (21%) and apply it to the 15,000 Aspergillosis hospitalizations per year, we come to $252M health care burden for just the cannabis associated hospitalized Aspergillus infections. This does not account for the 3.5X enrichment in this data for cannabis users. The entire cannabis lab testing market (all pathogens) is estimated to be $400M/year.
Untreated Aspergillosis is usually fatal. So for a $10-$100 cost for $45,000 worth of cannabis, we can dramatically reduce this risk and make the legal cannabis market differentiated from the black market.
But this all rests on labs that are all operating with equal sensitivity for the pathogen.
We do not have this yet as can be see in the Hawaii data. In fact, its not even clear which assays some regulators are running to make recalls on various product brands in California.
These California recalls are occurring and its still not clear to the public what methods the DCC is using to ascertain such recalls. This is going to result in Cannabis grows litigating or lobbying to remove the testing like in Oregon.
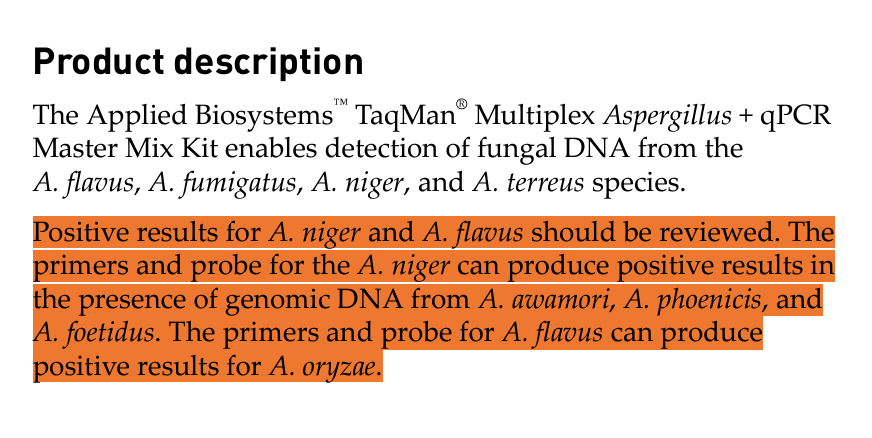
Rumor has it that the DCC is using a Thermo test that has not undergone AOAC certification. We have attempted to order this test to replicate and understand how the DCC might be regulating this industry and our Thermo reps can’t find the part number in their system. In other words, the assay is not up for public scrutiny and should never be used to issue a recall.
The product description of this test also demonstrates it has off target hits.
Conclusions
To perform a recall, they should be sequence validating the positive sample and ensure it is tested with high replication to rule out sampling issues. Ideally they would have their own lab capable of using all tests in the market place. The moment they decide on using a single test to address recalls, they will create a monopoly in that market as all labs will want to shift to the test the regulators are using. This is a subtle form of regulatory capture being deployed in the field, where regulators pick and chose which vendors they like and exclude the others. This is fine if some vendors are consistently failing as we can see in Hawaii but it otherwise is reducing competition in the marketplace and raises testing prices while lowering accuracy.
It will also expose these regulators to litigation if they demonstrate any bias in which vendors they support. They can only have preferences they can defend with data and with all of the public data emerging today, that picture is becoming more clear.
These data used in this analysis are from MCR labs public data release.
Addendum-
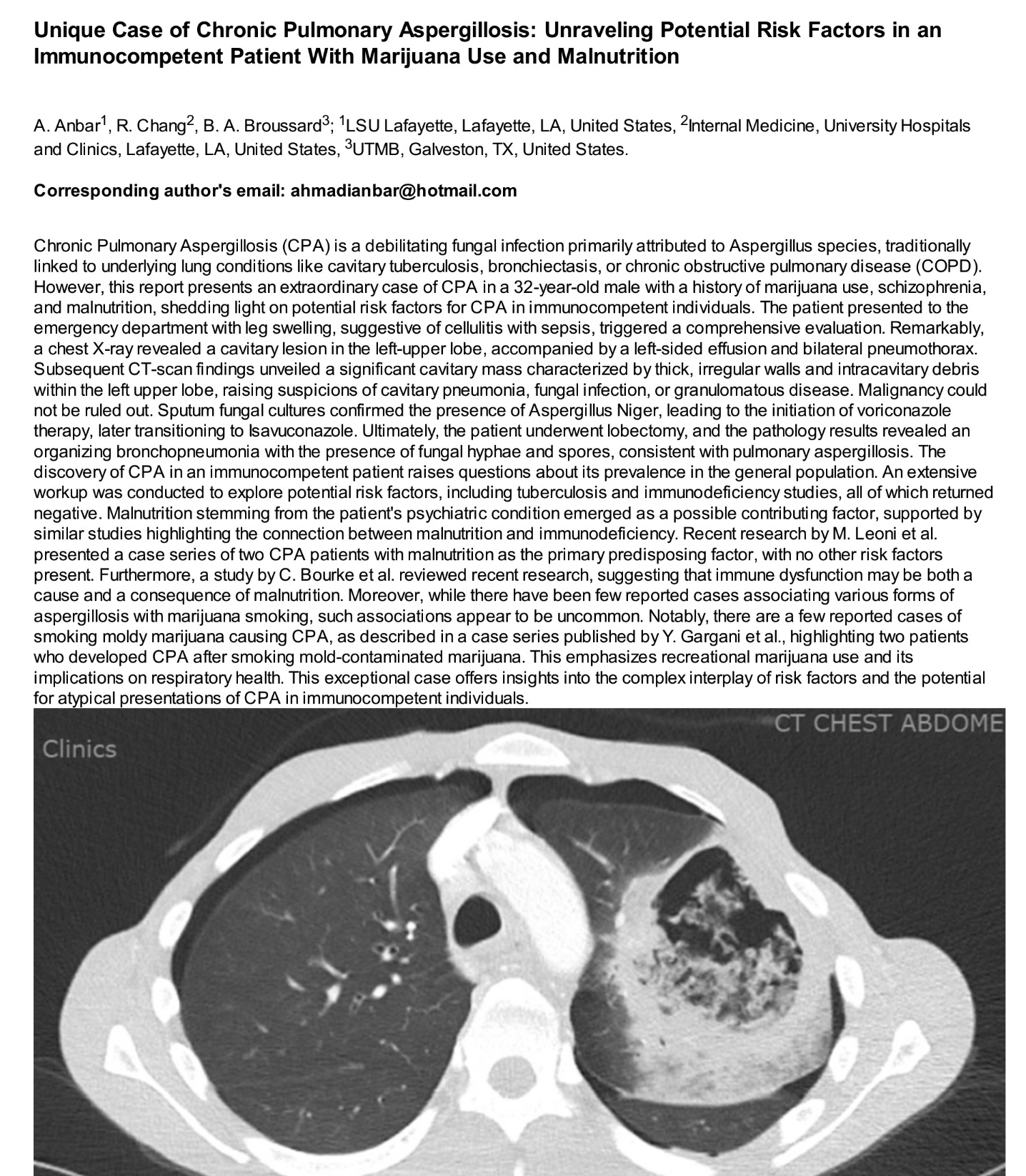
A May 2024 case report of Aspergillosis associated with Cannabis use. Lung Lobe removed.



Not a "pot" ingester in any form, but I found this article full of great points. Thanks.
This is illuminates a significant health consideration for all those who use marijuana as well the various other weeds which are sold for smoking purposes.