Rapid Boil Prep for assessing the dsDNA contamination in the Pfizer and Moderna mRNA vaccines
Preamble
Data challenging any scientific consensus that has been woven into a political fabric is frequently met with irrational hostility. In these circumstances it is prudent to make your hypothesis as easily reproducible as possible. When lives are at stake, waiting 6-12 months for peer review is irresponsible. You are gambling with 3 anonymous reviewers who will opine, emit half contemplated criticisms and occasional bromides, but never pick up a pipette to assess the veracity of the work.
Since we have made the mRNA vaccine DNA contamination findings public we have explored 9 different techniques to evaluate the scale of the contamination with varying results.
RNA-Seq
RNase-Seq
Qubit fluorometer
UV Spec (Nanodrop)
DNase- qPCR/RT-qPCR
RNase- qPCR/RT-qPCR
E.coli transformation
Agilent Tape Station (RNA and DNA tapes)
Oxford Nanopore
The only other work we can find on the sequence of these vaccines is Jeong et al. (Andrew Fire lab) and only the consensus sequence is published on Github. This is not peer reviewed anymore than our substack articles and unlike our study the raw reads are not public. It would be helpful to encourage the publication of these reads to assess if vector contamination is evident in prior assessments of these vaccines.
You will note, our release of these data on Substack has been met with a vocal minority of criticism on Twitter for not being peer reviewed. I cannot find similar criticisms for Andrew Fires work on these vaccines despite their labs omission of the raw read publication. This is likely because, the Fire lab didn’t report anything alarming but detection of vector sequences may have simply been overlooked due to the higher depths of coverage required to detect contaminants.
This selective outrage is incoherent but not uncommon during the hyper-polarized COViD pandemic. Modern day demands for peer review seem to be an anti-heresy weapon; a narrative control device to be deployed selectively and discriminatorily despite modern academia being pseudo-obsessed with diversity, equity and inclusion.
In such tumultuous environments, your time is best spent sharpening the tools that make your observation ever more obvious. We are not infallible, this work was conducted at a rapid pace with limited resources and the more eyes we have on this problem, the more we will learn. This is the true spirit of peer review and it’s largely been obfuscated with the centralized institutions that intermediate the review process attempting to monetize the information flow of science.
Introduction
DNA and RNA purification from Lipid Nanoparticles has previously been reported using variations on different Solid Phase Reversible Immobilization preparations (SPRI). These methods differ in their lysis reagents. One method used LiDs to dissolve the LNPs while the other used CTAB and Chloroform with centrifugation. Both methods require multiple steps and magnetic hardware to assess the vaccines nucleic acid content. These SPRI steps may be necessary for assays that demand high quality DNA/RNA such as E.coli transformation.
However, qPCR is known to work with simple boil preps in the agricultural and cannabis genomics space. This approach only requires the thermal cycler for sample prep and qPCR, thus limiting the laboratory hardware requirements for replication.
This process simply takes 1-10ul of the vaccine and dilutes it into a PCR compatible lysis buffer and heats it.
65C for 6 minutes
95C for 2 minutes
Results
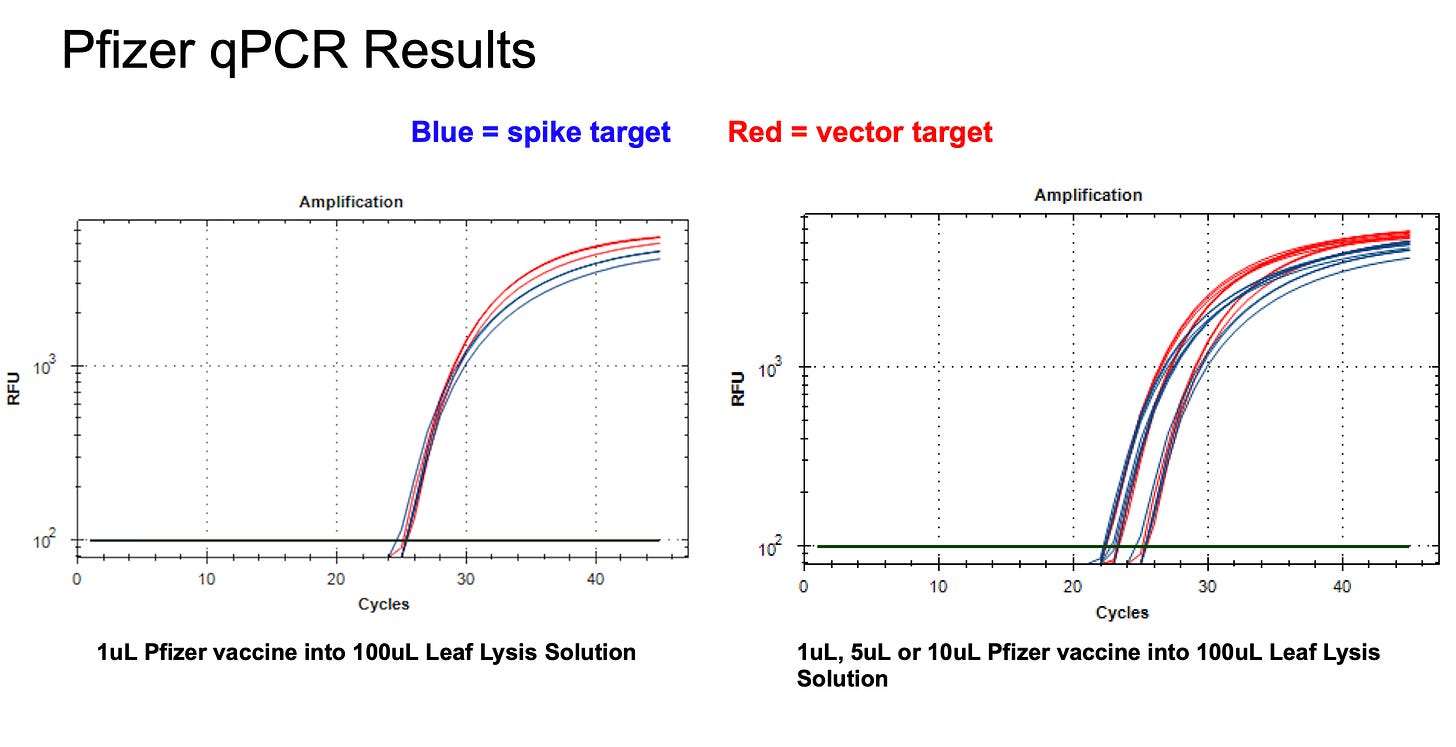
The CT scores from this method are delayed by a few CTs. This is expected as there is a 1:100 dilution (6 CT) putting the vaccine into the boil reagents.
The Pfizer Spike and Vector sequence present the same CT with qPCR as expected with an amplification that targets only DNA.
When RT-qPCR is performed we see an 8 CT jump (256 fold) in the Spike signal compared to the Vector. This is a bit larger of a delta CT than observed before on Pfizer vial 2.
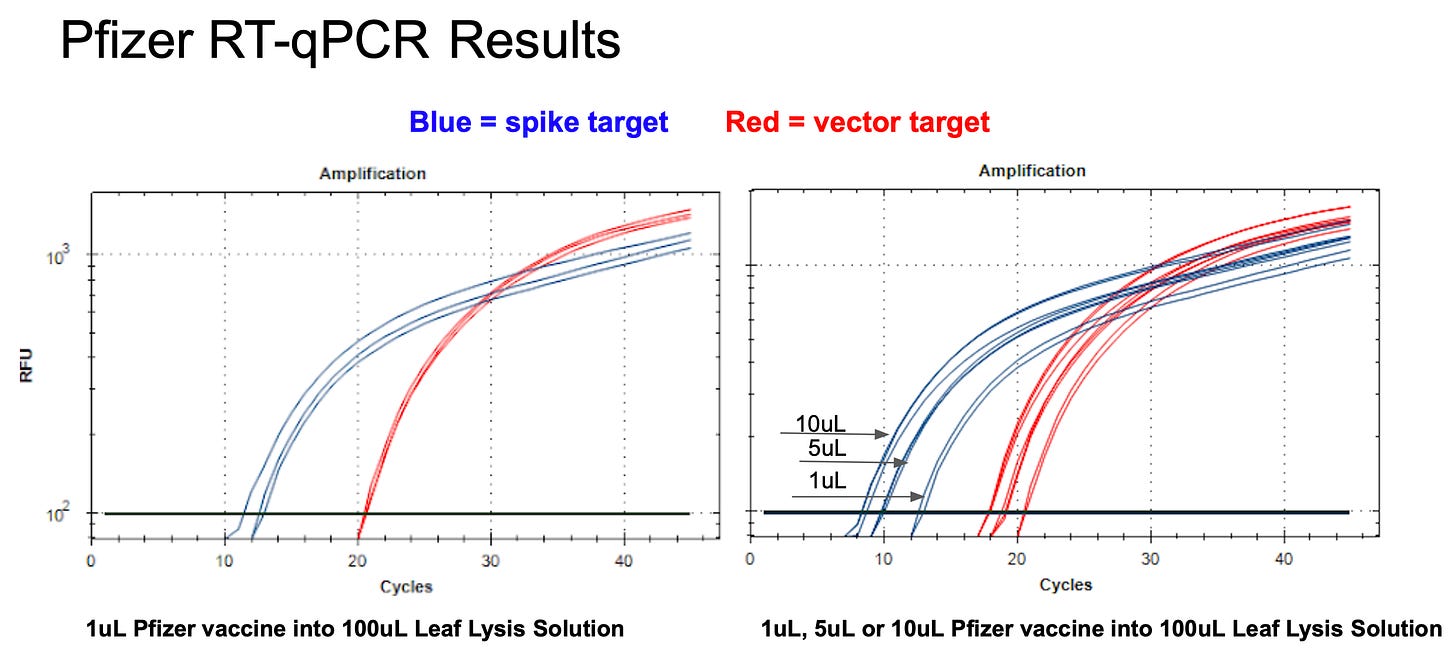
Note- prior work showed a Delta CT of 6 CT (64 fold seen in Blue vs Green on left figure 3 below) between Vector and Spike with RT-qPCR on Pfizer Vial 2.
Three things have changed since that work.
1)The current work is on Pfizer vial 1.
2)We have also increased the extension time to 1 minute for both qPCR and RT-qPCR as we noticed the RT-qPCR assays had lower efficiency at 30 second extensions and presented a poor slope. This reduced the accuracy of the Delta CTs as low efficiency qPCR often presents delayed CTs due to thresholding the poor slope of the assay. This change is more relevant to late CT quantitation.
3)The Vector assay has been migrated from Kan to the Origin of replication as the Kanamycin gene is different between Moderna and Pfizer and the Origins only differ by a single base.
To avoid confusion we only published the Vector-Origin set of Primers as the original Vector-Kanamycin primers do not accurately detect Moderna Vector.
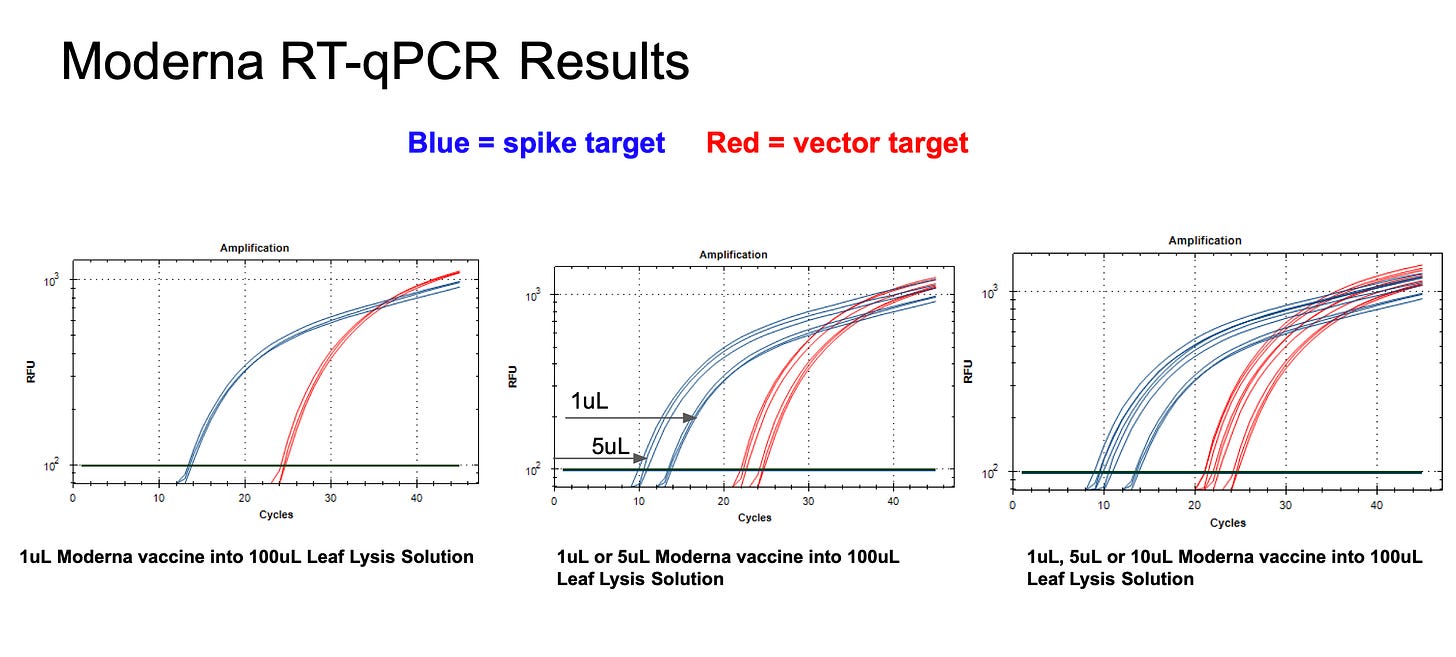
Moderna vial 1 shows a 5 CT offset between Spike and vector qPCR. This is a DNA only test and is different than what we see with Pfizer where the vector and spike qPCR CT are identical as expected.
This is important to keep in mind as we interpret the RT-qPCR results as it suggests, despite the improvement with the new Ori-vector primer pair on Moderna vaccines, we should observe equal CTs with DNA amplification of vector and spike. These should be equimolar at the DNA level.
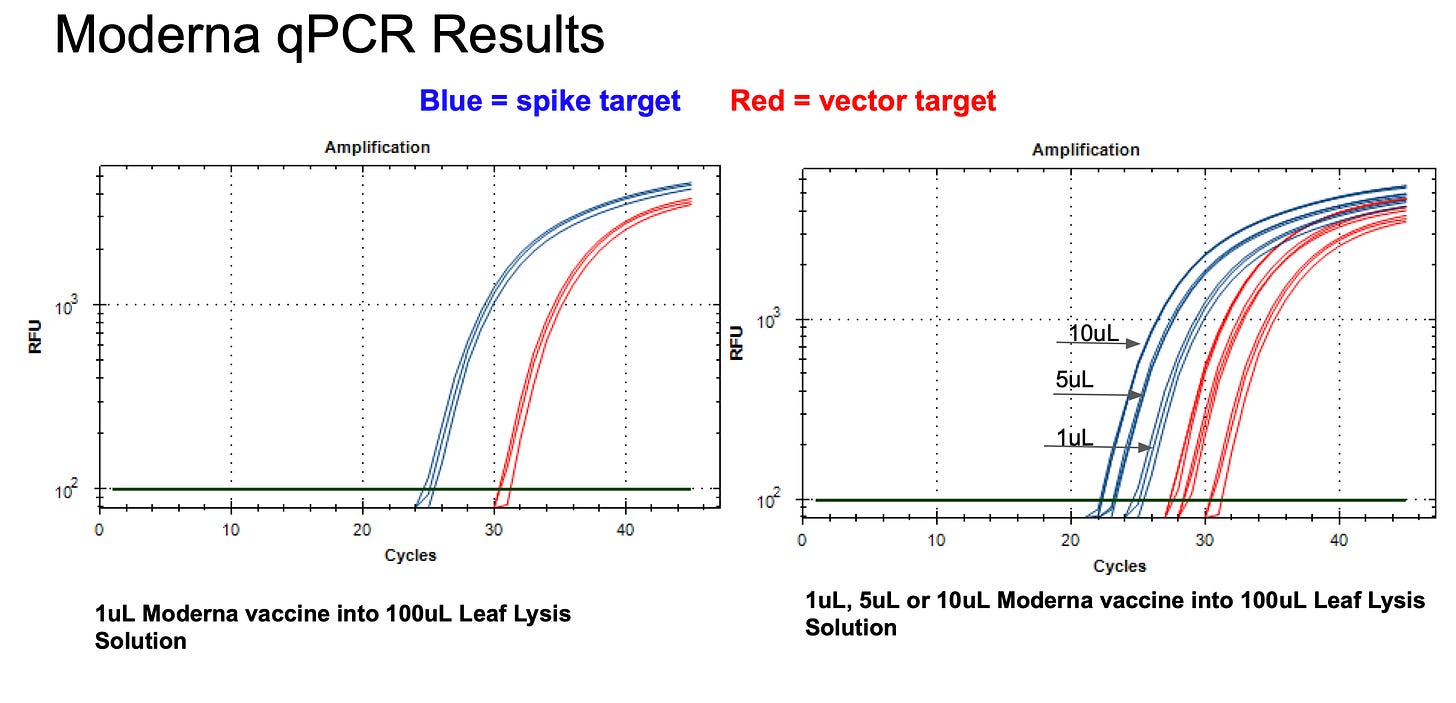
The RT-qPCR results with Moderna must consider that equimolar qPCR amplification of vector versus spike of Moderna DNA presents a 5 CT offset and Pfizers vector does not. Thus the 10 CT offset observed in the RT-qPCR data must be normalized to the qPCR offset 10CT - 5CT = 5CT difference in Spike versus vector. This represents 32 fold more RNA than DNA in Moderna vial 1.
Conclusions
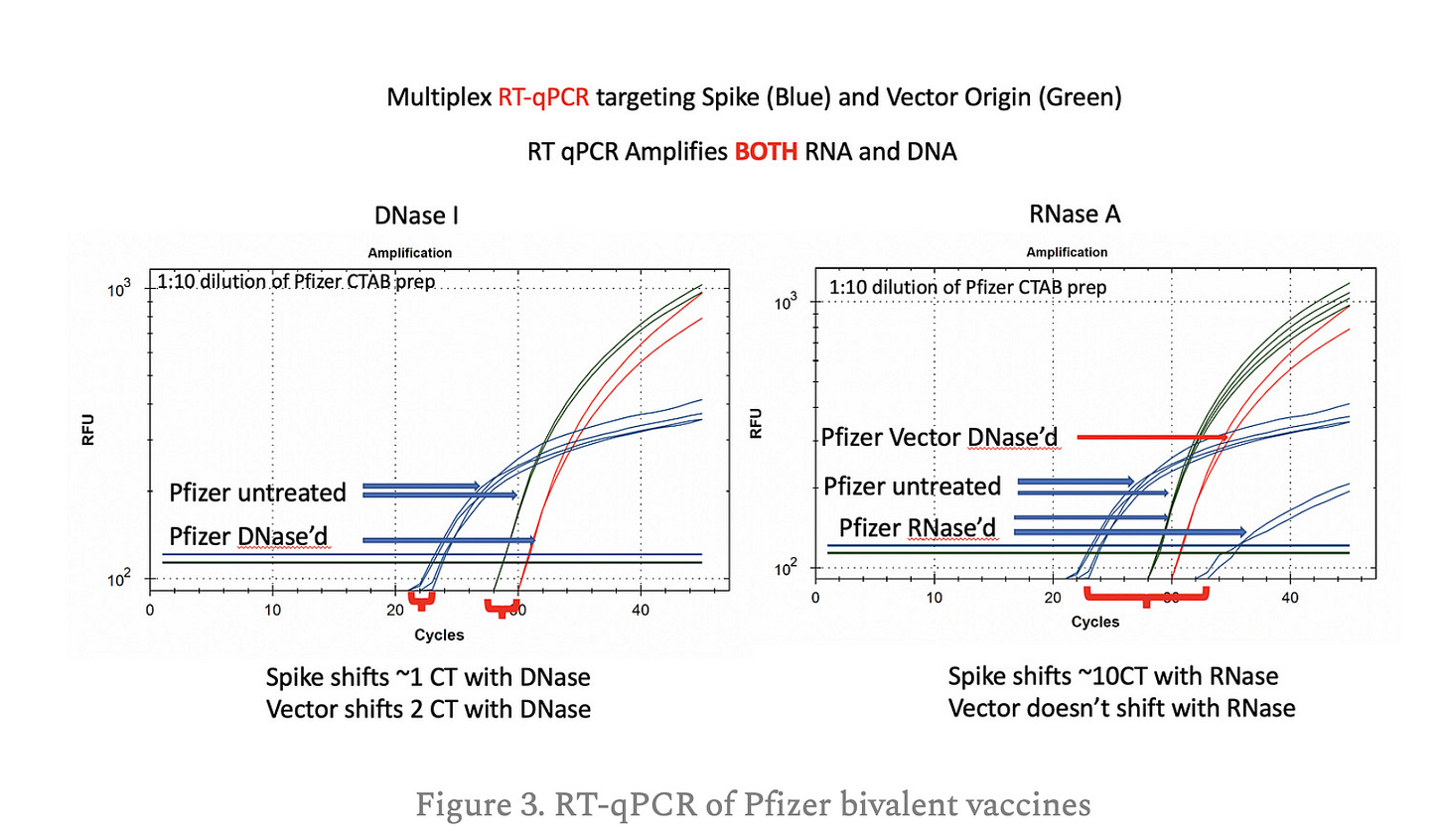
This boil prep represents a rapid and easy method to screen for DNA/RNA ratios in Pfizer and Moderna mRNA vials consuming as little as 1/300th of a dose. The test can be performed in under 2 hours with limited hands on time from a laboratory technician. The use of Non-template control qPCR can assure the user that the Vector and Spike signal is not coming from the lysis reagents and simplifies the verification of the source of the contamination. More vials should be surveyed to understand the vial to vial heterogeneity. The Moderna spike to vector CT offset with qPCR should be further scrutinized with gradient qPCR.
The RNase/DNase experiments in previous substacks showed more subtle CT shifts suggesting less of a difference between DNA and RNA but these DNase and RNase assays may not be run to completion or may have different rates of digestion with modified nucleotides. These DNase and RNase qPCR assays also need to be explored in different vials to further understand the lot to lot variance in DNA to RNA ratios.
Method notes
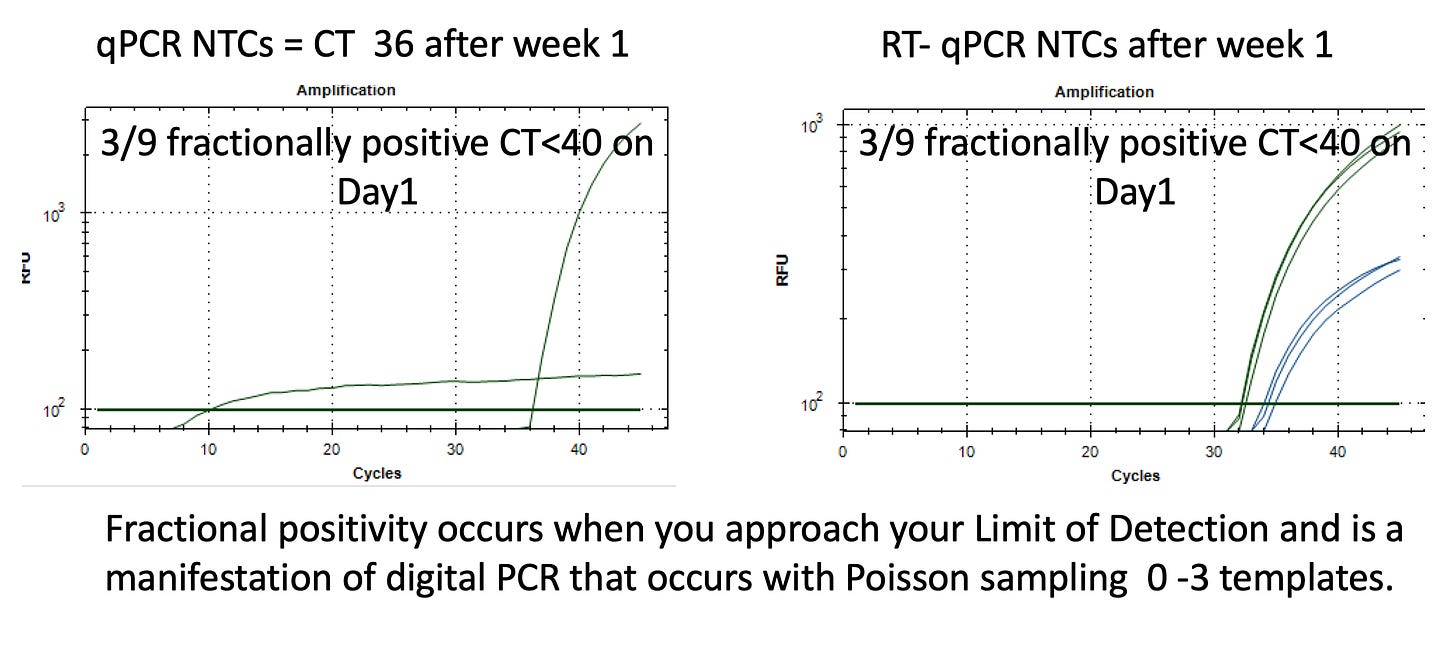
In our short experience handling these vaccines, we have noted a high rate of pipette contamination (NTCs creeping positive to CT 32) despite using filter tip pipettes. This is likely the result of handling highly concentrated nucleic acids that are encased in Lipid Nanoparticles. LNPs aerosolize easily upon negative pressure. Very analogously to how masks don’t stop aerosolized viruses, filter tip pipettes don’t stop aerosols. Pipette tip filters are much thicker than an N95 mask.
As a result, pay attention to the Non Template Control signals (NTCs). They will creep up on you after manipulating these samples for a week. Even with contamination, your vaccine signals will be 1000x higher (CTs in the early 20s) but this is good to catch early before it finds an expensive master mix vial. This becomes more pertinent with DNase and RNase studies where CTs past 32 are more scrutinized.



Here's some relevant info about the Pfizer vax and its true country of origin from an ex-employee at Pfizer's McPherson Kansas plant - https://rumble.com/v2dj152-full-episode-69-live-q-and-a-with-vsrf-founder-steve-kirsch-and-pfizer-whis.html at 28m30sec - and a report which corroborates a fairly long history of problems there:
"Pfizer has tapped its McPherson, Kansas, manufacturing plant for fill-finish work as it hustles to double its weekly COVID-19 vaccine output in the U.S. But the facility has a history of problems—and found itself in the FDA's crosshairs as recently as last January.
Pfizer’s McPherson plant was dinged by FDA inspectors for quality and cleanliness issues during a visit in late 2019 and early 2020, according to an inspection report obtained by Bloomberg via the Freedom of Information Act.
The plant, enlisted to fill and finish doses of Pfizer and BioNTech’s COVID-19 vaccine Comirnaty, has received a laundry list of complaints from the FDA since Pfizer acquired it as part of its Hospira buyout in 2015. Pfizer has repeatedly vouched for remediation efforts there, and “as of today, the McPherson site’s plan remains on track, with nearly all of the improvements completed,” Eamonn Nolan, senior manager of global media relations at Pfizer, said via email.
The 2019-20 inspection found that the plant had released drugs without reviewing quality issues flagged during routine testing. Inspectors also turned up mold and bacteria in areas that were meant to be sterile and said the plant neglected to properly sample drugs for excessive levels of certain toxins, Bloomberg reported.
Specifically, the McPherson plant failed to adequately test for endotoxins—which are created by bacteria like Escherichia coli or botulism—in drugs such as morphine and the cancer med Nivestym." https://www.fiercepharma.com/manufacturing/pfizer-s-mcpherson-plant-filling-covid-19-vaccine-dinged-for-repeat-offenses-last
You said: "This is likely the result of handling highly concentrated nucleic acids that are encased in Lipid Nanoparticles. LNPs aerosolize easily upon negative pressure. Very analogously to how masks don’t stop aerosolized viruses, filter tip pipettes don’t stop aerosols. Pipette tip filters are much thicker than an N95 mask."
I presume you have seen this?
https://pubmed.ncbi.nlm.nih.gov/35082653/